Phenylethanoid glycoside extract from acanthus ilicifolius, and preparation method and application thereof as anti-liver injury medicine
A technology of phenethyl glycosides and ethyl alcohol glycosides is applied in the application of anti-liver injury drugs, the field of phenethyl alcohol glycosides extract from rat sclerotium and its preparation, which can solve the problems of side effects, limited therapeutic effect and the like, and achieve the improvement of liver lesions. , the effect is obvious, the preparation method is simple
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] 1. Preparation of mouse boarphenylethanol glycosides extract
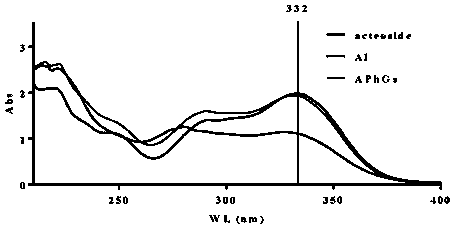
[0027] Grind the whole plant of dried bougainvillea, pass through a 20-30 mesh sieve to obtain the powdered bougainvillea, add 1kg of powder to 10kg of 95% ethanol solution, soak for 2 hours, reflux for 3 hours, collect the extract by filtration, and use 8kg of Reflux extraction with 95% ethanol solution for 1.5h, combine the two extracts, concentrate and go through D101 macroporous resin column chromatography, elute with 10%, 30%, and 50% ethanol solution in sequence, and collect 4 column volumes for each gradient , collecting fractions obtained by eluting with 50% ethanol solution, concentrating and drying to obtain the phenylethanol glycosides extract.
[0028] 2. Identification of mouse boar phenylethanol glycosides extract
[0029] The ultraviolet spectrum of phenylethanol glycosides is characterized by the maximum absorption at 332nm. Therefore, each eluate component is scanned with 200-400nm ultravi...
Embodiment 2
[0032] CCl 4 Pharmacodynamic studies in an induced mouse liver injury model.
[0033] 1 Experimental method
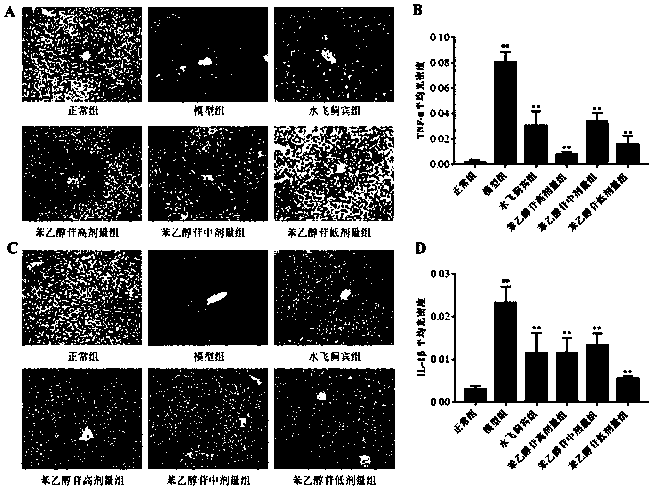
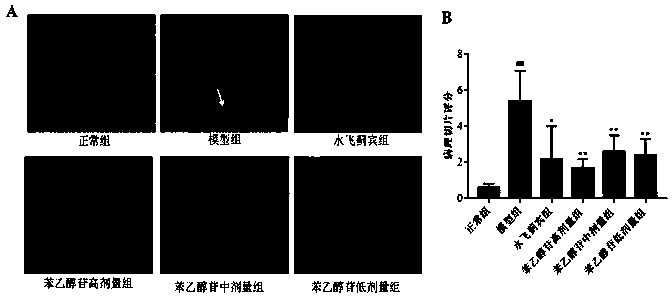
[0034]48 healthy C57 mice, SPF grade, male, weighing 20-22g, were provided by Hubei Experimental Animal Research Center, batch number: NO.42000600033017. The above-mentioned 48 mice were divided into 6 groups on average at random, and intraperitoneal injection was given to the phenylethanol glycosides extract of the present invention (phenylethanol glycoside group), and the dosage was respectively 300 mg / kg (high dose group), 150 mg / kg (middle dose group). ), 75mg / kg (low dose group); silibinin group (150mg / kg); normal group and model group are normal saline. 1 time / d, continuous administration for 3 days. 2 hours after the last administration, the normal group was intraperitoneally injected with 15 μL / mouse of olive oil, and the other groups were injected with 15 μL / mouse of 50% CCl 4 (Olive oil diluted 1:1). After 8 hours of anesthesia, the heart was punctured t...
Embodiment 3
[0068] CCl 4 Pharmacodynamic study of induced L-02 hepatocyte injury.
[0069] 1 Experimental method
[0070] L-02 hepatocytes were provided by the China Center for Type Culture Collection. L-02 hepatocytes were cultured in a culture medium containing 1640 complete culture solution and DMEM complete culture solution at 37°C and 5% CO 2 Cultured at constant temperature, harvested cells in the exponential growth phase, prepared into 1×10 6 cells / mL of the suspension, and then add 100 μL of this suspension to a 96-well plate. Grouped into: normal group, negative control group, CCl 4 Model group, positive control group (silibinin), phenylethanol glycoside group (high, middle and low dose group). After 24 hours of incubation, cells in the experimental and positive groups were treated with different concentrations of phenylethanol glycosides and silibinin. CCl 4 The same amount of DMSO was added to the model group and the negative control group. Five replicates were performed...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com