A kind of isoxazoline compound and use thereof
A technology of isoxazoline and compound, applied in application, organic chemistry, biocide and other directions, can solve problems such as herbicide resistance, and achieve the effect of outstanding killing activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
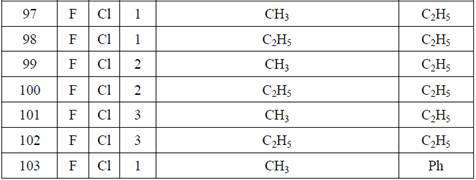
Examples
Embodiment 1
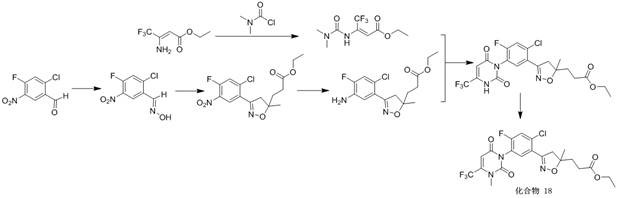
[0085] Example 1 Preparation of compound 18
[0086]
[0087] 1) Preparation of 2-chloro-4-fluoro-5-nitrobenzaldehyde oxime
[0088] Dissolve 42 g (0.206 mol) of 2-chloro-4-fluoro-5-nitrobenzaldehyde in 200 ml of ethanol, lower it to 0 °C, add 17.4 g (0.25 mol) of hydroxylamine hydrochloride aqueous solution dropwise with stirring, and then rise to The reaction was stirred at room temperature. After 2 hours, the reaction was complete by TLC monitoring. Poured into water, filtered to obtain 38.3 g of white solid. Melting point 103 ℃.
[0089] 2) Preparation of ethyl 3-(3-(2-chloro-4-fluoro-5-nitrophenyl)-5-methyl-4,5-dihydroisoxazol-5-yl)propanoate
[0090] Dissolve 43.7g (0.2mol) of 2-chloro-4-fluoro-5-nitrobenzaldehyde oxime in 150ml N,N - In dimethylformamide, the temperature was raised to 30 °C, and 32 g (0.24 mol) of NCS was added in batches at this temperature to form a pale yellow solution, which was kept at 35 °C for 1 hour. Cool to room temperature, add 300 ml...
Embodiment 2
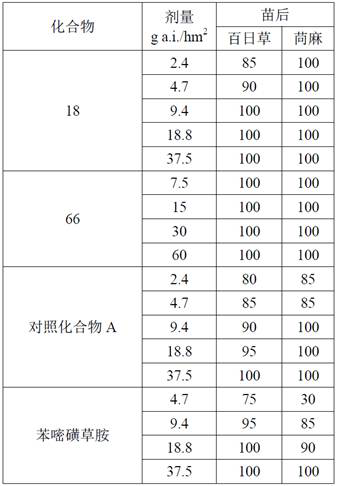
[0104] Example 2 Determination of indoor herbicidal activity
[0105] The herbicidal activity test method of the compounds of the present invention is as follows:
[0106]Quantitative amounts of grass weeds (barnyard grass, golden foxtail grass) and broadleaf weeds (zinnia, abalone) seeds were sown in paper cups with a diameter of 7 cm containing nutrient soil, covered with soil 1 cm after sowing, and suppressed. , After being drenched in water, cultured in the greenhouse according to the conventional method. Grass weeds grow to the 2~3 leaf stage, broad-leaved weeds are 2~4 leaf stage stem and leaf spray treatment; pre-emergence soil spray treatment is carried out 24 hours after sowing. According to the experimental design dose, spray treatment on a crawler crop sprayer (designed and manufactured by Engineer Research Ltd., UK) (spray pressure 1.95 kg / cm2) 2 , the spray volume is 500 L / hm 2 , the crawler speed is 1.48km / h). The experiment was repeated 3 times. After treat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com