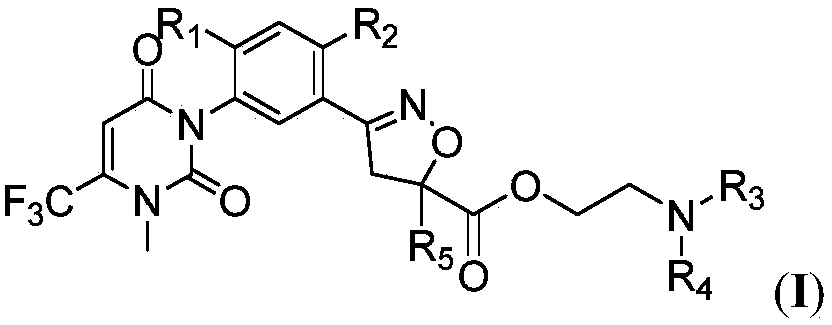
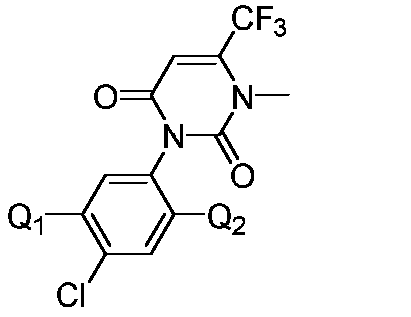
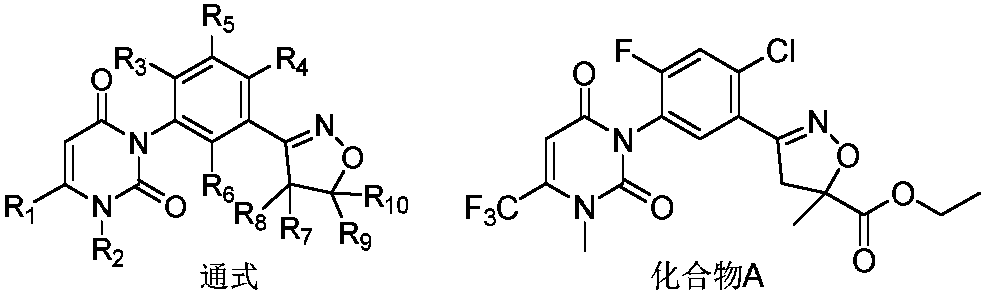
Isooxazoline carboxylate compound and application
A technology of isoxazoline carboxylic acid and ester compounds, applied in the field of agricultural herbicides, can solve the problems of satisfactory weed control effect and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0081] The preparation of embodiment 1 compound 1
[0082]
[0083] 3-(2-Chloro-4-fluoro-5-(3-methyl-2,6-dioxo-4-trifluoromethyl-3,6-dihydropyrimidin-1(2H)-yl)- Phenyl)-5-methyl-4,5-dihydroisoxazole-5-carboxylic acid (preparation with reference to CN105753853) (0.45g, 0.001mol) was added to a reaction flask with 20ml of dichloromethane, stirred at room temperature Next, dimethylaminoethanol (0.11g, 0.0012mol), DMAP (0.13g, 0.001mol) and EDCI (0.29g, 0.0015mol) were added successively, and the reaction mixture was stirred overnight at room temperature, 100ml of water was added, and washed with ethyl acetate (100ml*2) extraction, the organic phases were combined and dried over anhydrous magnesium sulfate, filtered and desolvated, and column chromatography (ethyl acetate:petroleum ether=1:5) gave 0.19g of oily compound 1.
[0084] 1 H-NMR (300MHz, internal standard TMS, solvent CDCl 3 )δ(ppm): 1.68(s,3H), 2.30(s,6H), 2.62(t,2H), 4.26(t,2H), 3.38(d,1H), 3.60(s,3H), 3.90( d,...
Embodiment 2
[0087] Embodiment 2 Indoor herbicidal activity assay
[0088] The herbicidal activity testing method of compound of the present invention is as follows:
[0089] Quantitative gramineous weeds (barnyardgrass, golden foxtail) and broad-leaved weeds (zinnia, velvetleaf) were sown in paper cups with a diameter of 7 cm and filled with nutrient soil, and covered with 1 cm of soil after sowing. After watering, they were cultivated in the greenhouse according to conventional methods. Gramineae weeds grow to 2-3 leaf stage, and broad-leaved weeds 2-4 leaf stage stem and leaf spray treatment; pre-emergence soil spray treatment is carried out 24 hours after sowing. According to the test design dose, carry out the spray treatment (spray pressure 1.95kg / cm2) on the crawler crop sprayer (Engineer Research Ltd. design and production, UK). 2 , spray volume 500L / hm 2 , crawler speed 1.48km / h). The experiment was repeated 3 times. After treatment, the test materials are placed in the opera...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com