Method for removal of impurities from bacterial capsular polysaccharide based preparations
A technology of polysaccharide and polysaccharide solution is applied in the directions of antibacterial drugs, medical preparations containing active ingredients, pharmaceutical formulas, etc., and can solve the problems of time-consuming, expensive, and complicated process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
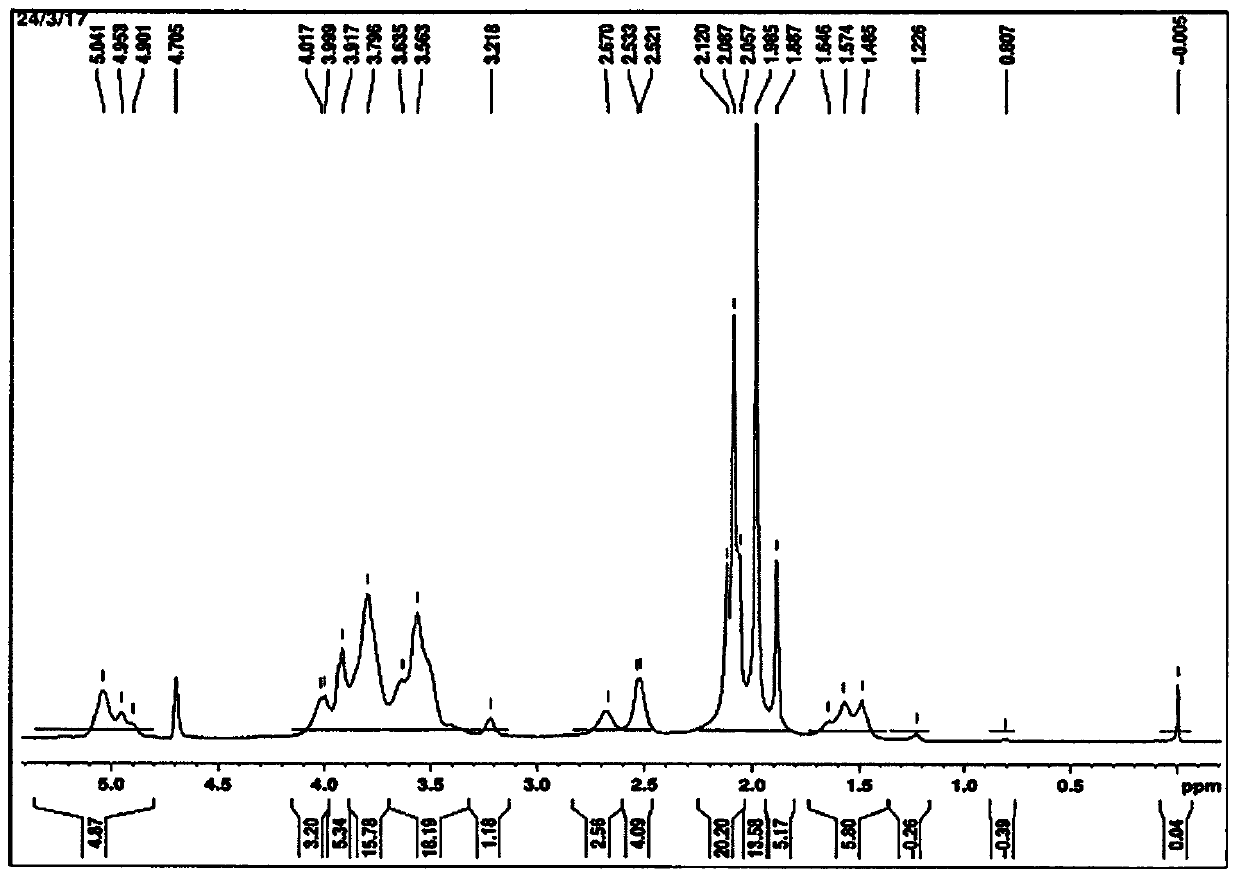
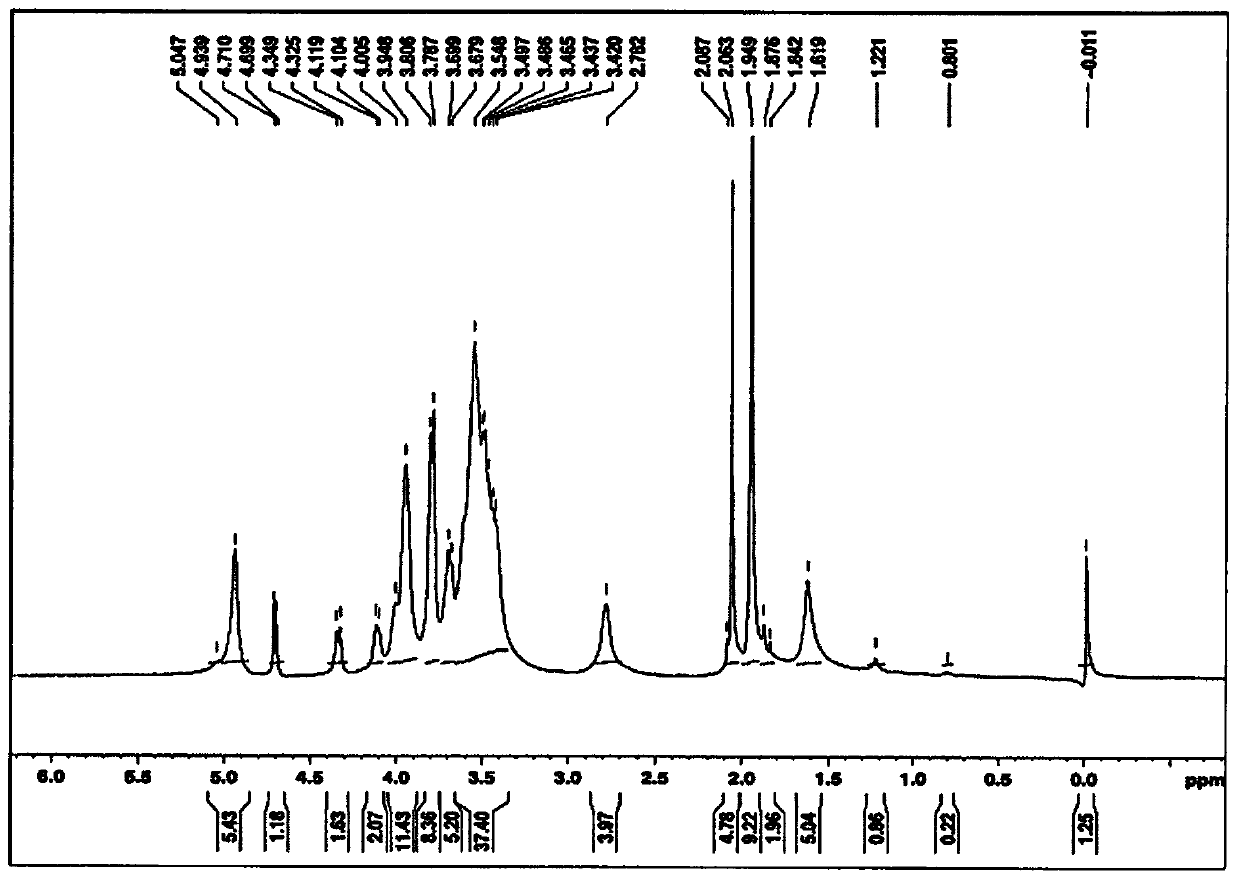
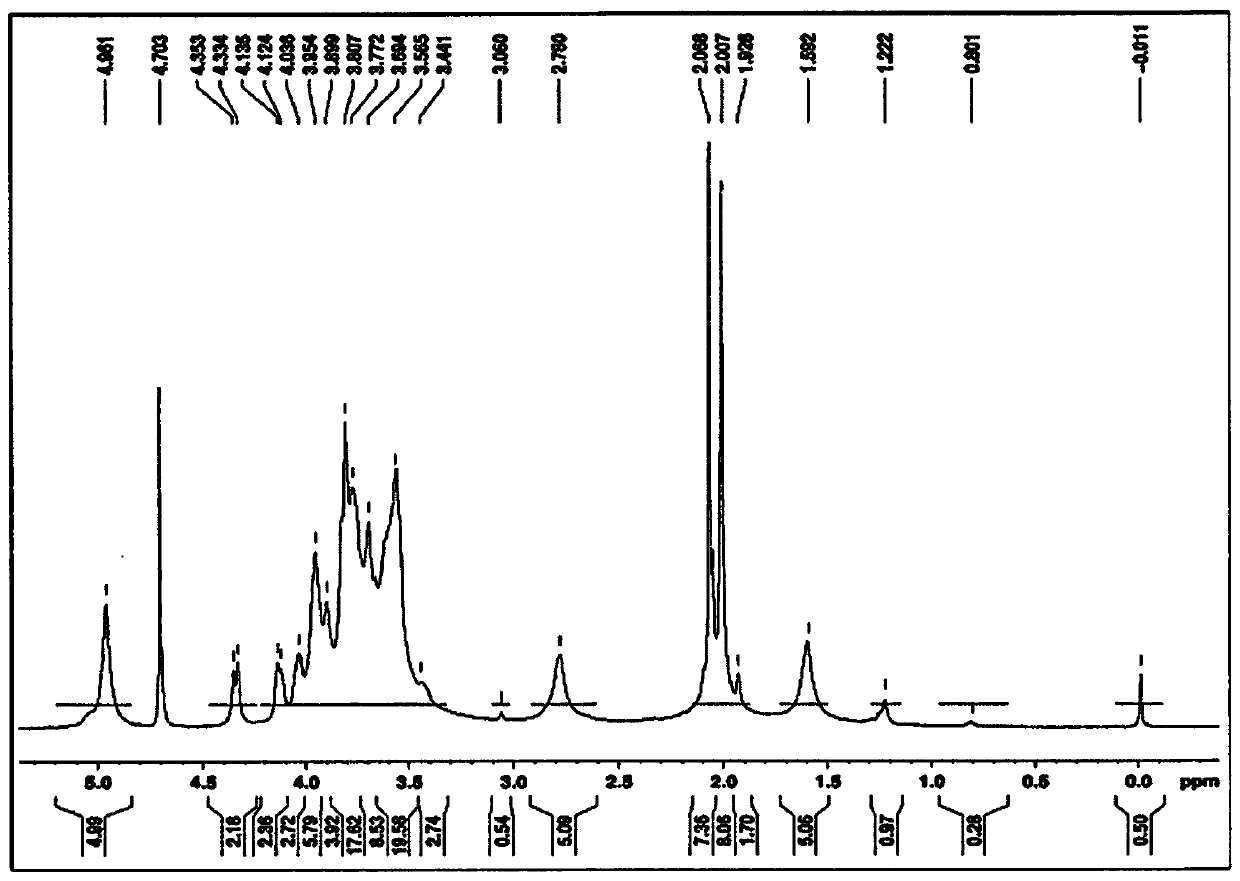
[0043] According to a first embodiment of the invention, centrifugation is used to separate and purify bacterial capsular polysaccharides from the inactivated harvest. The supernatant was diafiltered with a 100 kD tangential flow filtration unit. It is very clear to a person skilled in the art that instead of centrifugation and diafiltration any other suitable method can be used to concentrate bacterial capsular polysaccharides. In one of the preferred aspects of this embodiment, the capsular polysaccharide is derived from Neisseria meningitidis serogroups A, C, W, Y and X.
[0044] According to a second embodiment of the invention, the retentate obtained in the first embodiment is treated with an anionic surfactant / detergent. Anionic detergents are selected from the group comprising alkyl sulphates, sodium lauryl sulphate, sodium deoxycholate, sodium dodecyl sulphate, sodium s-alkyl sulphate , fatty alcohol polyoxyethylene ether sodium sulfate, sodium oleyl sulfate, N-oleyl...
no. 3 approach
[0047] According to a third embodiment of the present invention, a strong base is added to the mixture obtained in the above embodiment, and the pH of the strong base is adjusted to be between pH9 and pH11 with continuous stirring at room temperature for 1 hour. The strong base is selected from the group comprising sodium hydroxide, potassium hydroxide, sodium carbonate, hydroxylamine, triethylamine and lithium hydroxide.
[0048] According to a preferred aspect of the third embodiment of the present invention, the strong base, i.e. sodium hydroxide, with a final concentration between 5M and 20M is added to the mixture obtained by the above embodiment, and the mixture is stirred at room temperature for 1 hour to dissolve the The pH of the strong base, sodium hydroxide, was adjusted to pH 10.5.
[0049] In another aspect of the third embodiment of the present invention, instead of adding base, EDTA and sodium acetate are added to the mixture obtained from the second embodiment,...
no. 10 approach
[0057] According to the tenth embodiment of the present invention, the solution obtained from the above embodiment is fully diafiltered through WFI (Water for Injection) using 100 kDa tangential flow filtration, and passed through a 0.2μ filter, and heated at -20°C or -20 ℃ below as the final bulk storage.
[0058] According to the eleventh embodiment of the present invention, the purified capsular polysaccharides of Neisseria meningitidis serogroups C, W and Y are obtained according to the following steps:
[0059] • N. meningitidis serogroups C, W and Y were grown separately in appropriate media and inactivated using formaldehyde.
[0060] • Add sodium lauryl sulfate to the inactivated harvest to a final concentration of 1%, and stir at room temperature for 2 hours.
[0061] • Add sodium hydroxide until the final concentration is 05mM-20mM, and adjust the pH to pH 10.5 with constant stirring at room temperature for 1 hour.
[0062] - Neutralize the solution obtained from t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap