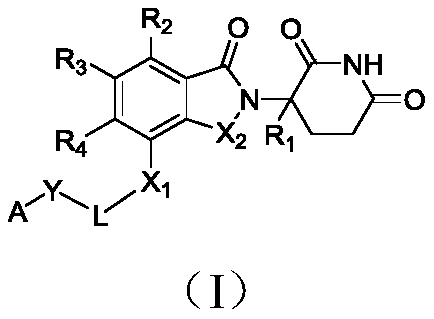
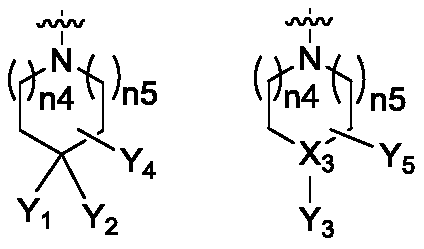
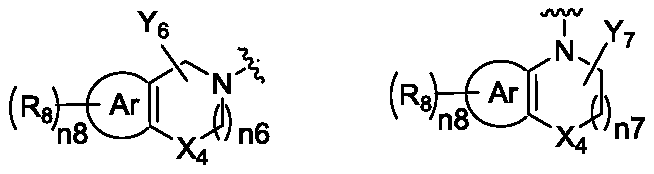
Isoindoline compound, and preparation method, pharmaceutical composition and application thereof
A compound and prodrug technology, applied in the field of polysubstituted isoindoline compounds, can solve the problems that are still in preclinical or clinical research, and the effect of other indications is not satisfactory.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0480] Example 1: 3-(1-oxo-4-(5-(quinoline-4-oxo)pentyl)isoindoline-2-)piperidine-2,6-dione (1)
[0481]
[0482] 3-(4-(5-hydroxypentyl)-1-oxoisoindoline-2-)piperidine-2,6-dione (100mg, 0.303mmol, 1eq.), 4-hydroxyquinoline (132mg, 0.909mmol, 3eq.) and triphenylphosphine (159mg, 0.605mmol, 2eq.) were dissolved in 20mL dry THF, and diisopropyl azodicarboxylate (120μL, 0.605mmol, 2eq. ). The resulting mixture was stirred and reacted at room temperature for 2 hours. After the reaction was complete, the solvent was removed under reduced pressure. The resulting residue was first separated by silica gel column chromatography, and then purified by HPLC to obtain 3-(1-oxo-4-(5-( Quinoline-4-oxo)pentyl)isoindoline-2-)piperidine-2,6-dione 52 mg, white solid, yield 38%; 1 H NMR (400MHz, DMSO) δ11.00(s, 1H), 8.71(d, J=5.2Hz, 1H), 8.12-8.07(m, 1H), 7.93(dd, J=8.4, 0.5Hz, 1H) , 7.73(ddd, J=8.4, 6.9, 1.5Hz, 1H), 7.57(dd, J=7.3, 1.3Hz, 1H), 7.55-7.51(m, 1H), 7.48(dd, J=7.5, 1.4Hz , 1H),...
Embodiment 2
[0483] Example 2: 3-(1-oxo-4-(3-(quinoline-4-oxo)propyl)isoindoline-2-)piperidine-2,6-dione (2)
[0484]
[0485] Under nitrogen protection, 3-(4-(3-hydroxypropyl)-1-oxoisoindoline-2-)piperidine-2,6-dione (48mg, 0.16mmol), 4-hydroxy Quinoline (70mg, 0.48mmol) and triphenylphosphine (84mg, 0.32mmol) were added to a 100mL round bottom flask, 20mL of tetrahydrofuran was added, and vigorously stirred. Then diisopropyl azodicarboxylate (65 mg, 0.32 mmol) was added. After the reaction was completed, the solvent was spun away, and purified by HPLC to obtain 17.6 mg of the product, with a yield of 26%; 1H NMR (400MHz, DMSO) δ10.96(s, 1H), 9.10(d, J=6.4Hz, 1H), 8.26(d, J=7.7Hz, 1H), 8.13(d, J=8.4Hz, 1H ), 8.08-8.01(m, 1H), 7.79(t, J=11.3Hz, 1H), 7.56(t, J=6.4Hz, 2H), 7.46(dd, J=10.5, 4.4Hz, 2H), 5.11 (dd, J=13.3, 5.1Hz, 1H), 4.52(t, J=5.9Hz, 2H), 4.47(d, J=17.1Hz, 1H), 4.31(d, J=17.1Hz, 1H), 3.00 -2.84(m, 3H), 2.6(m, 1H), 2.36-2.14(m, 3H), 1.97-1.86(m, 1H).
Embodiment 3
[0486] Example 3: 3-(1-oxo-4-(6-(quinoline-4-oxo)hexyl)isoindoline-2-)piperidine-2,6-dione (3)
[0487]
[0488] Replace 3-(4-(5-hydroxypentyl)-1-oxoisoindoline-2-)piperidine-2,6-dione with 3-(4-(6-hydroxyhexyl)-1 -Oxoisoindoline-2-)piperidine-2,6-dione, the preparation method is the same as 3-(1-oxo-4-(5-(quinoline-4-oxo)pentyl)isoindoline Indoline-2-)piperidine-2,6-dione, 47.2 mg, yield 50%; 1 H NMR (400MHz, DMSO) δ11.00(s, 1H), 8.72(d, J=5.3Hz, 1H), 8.14(dd, J=8.3, 0.9Hz, 1H), 7.97-7.91(m, 1H) , 7.74(ddd, J=8.4, 6.9, 1.5Hz, 1H), 7.56(tdd, J=4.7, 3.9, 1.2Hz, 2H), 7.48-7.40(m, 2H), 7.02(d, J=5.3Hz , 1H), 5.13(dd, J=13.2, 5.2Hz, 1H), 4.46(d, J=17.2Hz, 1H), 4.30(d, J=17.1Hz, 1H), 4.25(t, J=6.3Hz , 2H), 2.92(ddd, J=17.5, 13.5, 5.5Hz, 1H), 2.70-2.63(m, 2H), 2.59(dd, J=16.3, 2.0Hz, 1H), 2.41(ddd, J=26.5 , 13.4, 4.6Hz, 1H), 2.05-1.95(m, 1H), 1.92-1.83(m, 2H), 1.71-1.62(m, 2H), 1.61-1.51(m, 2H), 1.44(dt, J =15.9,8.0Hz,2H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com