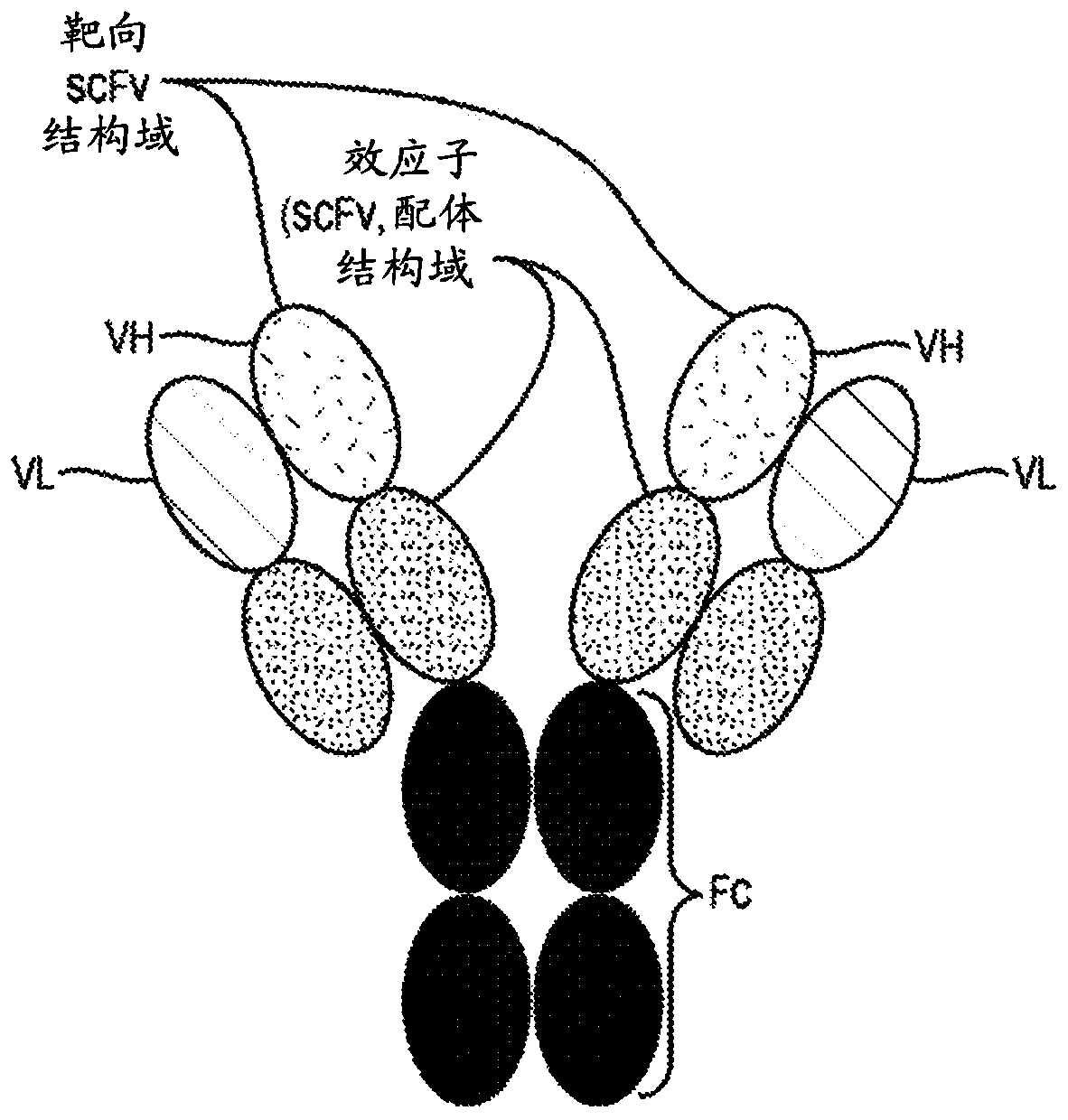
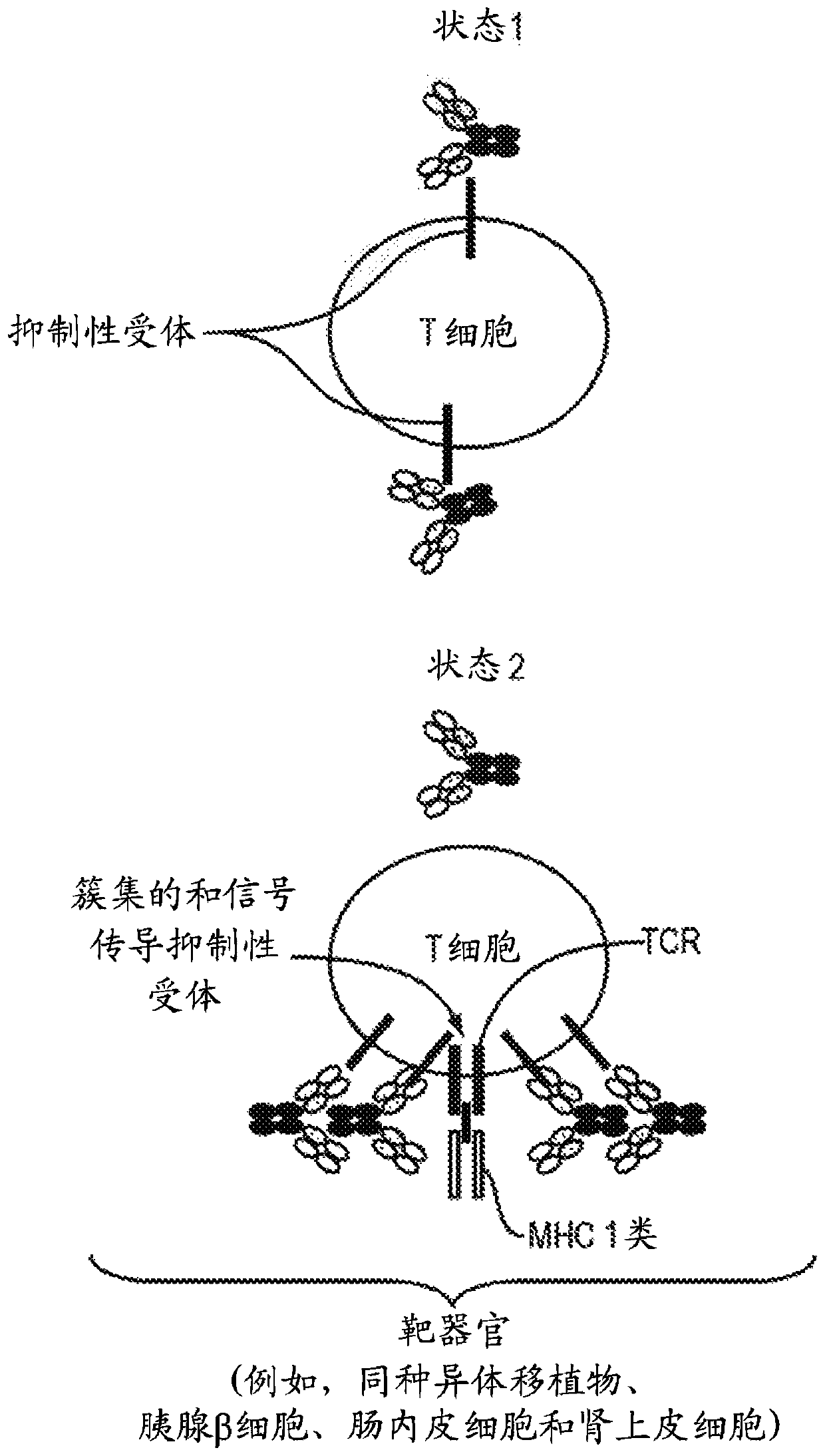
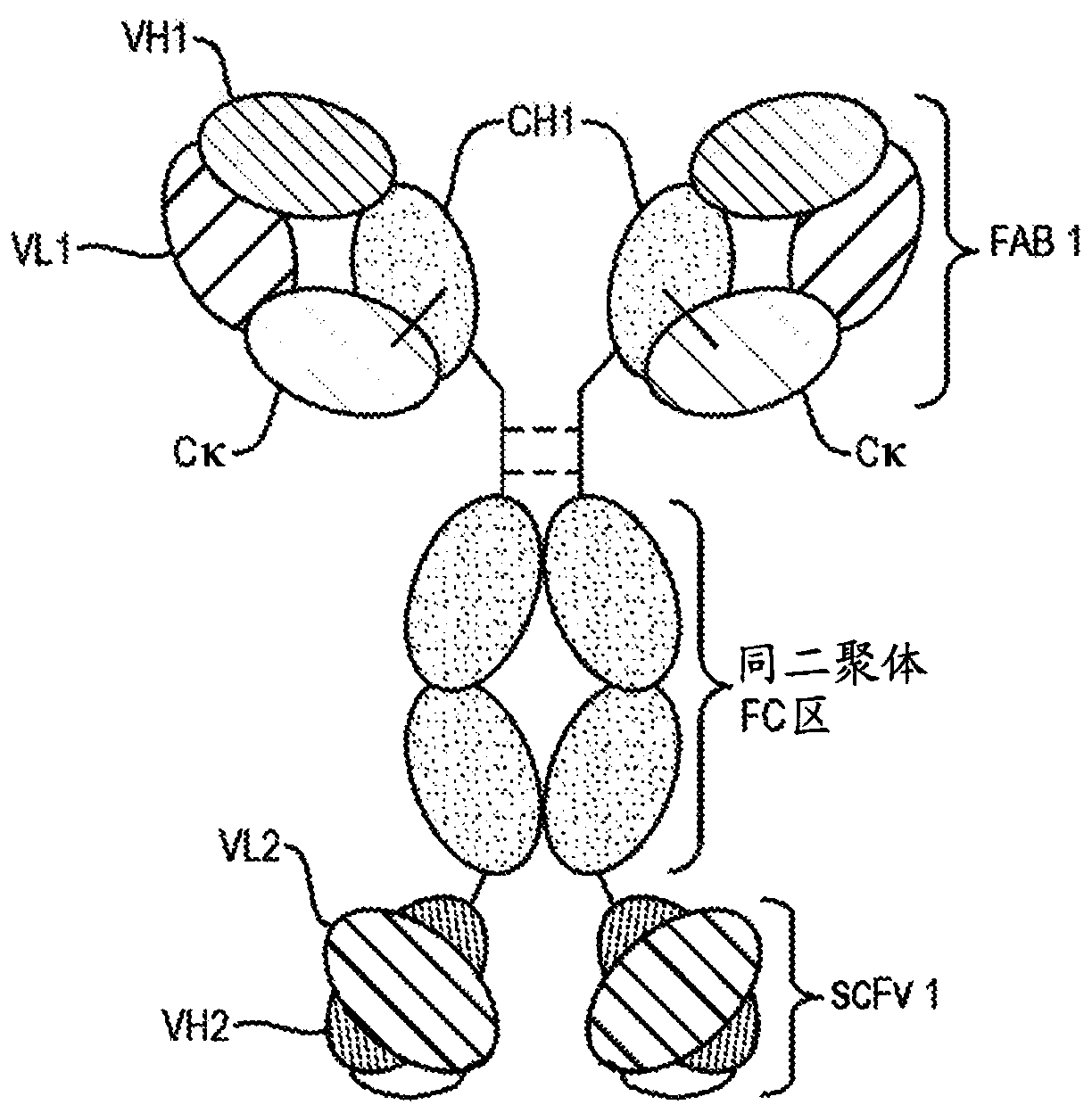
Targeted immunotolerance
A technology targeting parts and immune cells, applied in immunoglobulin, targeting specific cell fusion, allergic diseases, etc., can solve side effects and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[1076] Example 1: Agonizing HLA-targeted PD-1 of therapeutic compounds.
[1077] Engineering of PD-1 Agonist Therapeutics Targeting HLA
[1078] Binding domains specific for HLA-A2 were obtained by cloning the variable regions of Ig heavy and light chains from BB7.2 hybridoma (ATCC) and converting into single chain Ab (scFv). The activity and specificity of the scFv can be confirmed by assessing the binding of BB7.2 to cells expressing HLA-A2 compared to cells expressing other HLA-A alleles. The minimal PD-L1 residues required for PD-1 binding activity were identified by systematically evaluating the 3′ and 5′ amino acid requirements of the PD-L1 IgV domain corresponding to amino acids 68–114. The expression construct was designed, and the protein was synthesized and purified, and the PD-1 binding activity was tested by Biacore. The minimum essential amino acids required for PD-1 binding by the PD-L1 IgGV domain are referred to as PD-L1-IgV. To generate BB7.2 scFv and PD-L1...
Embodiment 2
[1082] Example 2: CD39 and / or CD73 as effector domains generate purinergic halides surrounding target cell types or tissues
[1083] Catalytically active fragments of CD39 and / or CD73 are fused to the targeting domain. After binding and accumulation at the target site, CD39 phosphohydrolyzes ATP to AMP. After binding and accumulation at the target site, CD73 dephosphorylates extracellular AMP to adenosine. Soluble catalytically active forms of CD39 suitable for use herein have been found to circulate in human and murine blood, see eg Yegutkin et al. FASEB J. 2012 Sep;26(9):3875-83. Soluble recombinant CD39 fragments are also described in Inhibition of platelet function by recombinant soluble ecto-ADPase / CD39, Gayle, et al., J Clin Invest. 1998 May 1;101(9):1851-1859. Suitable CD73 molecules include soluble forms of CD73 that can be shed from endothelial cell membranes by proteolytic cleavage or by hydrolysis of the GPI anchor by shear stress, see e.g. Refs: Yegutkin G, Bodin...
Embodiment 3
[1085] Example 3: Measuring antibody-induced PD-1 signaling.
[1086] Jurkat cells stably express 2 constructs, 1) human PD-1 polypeptide fused to b-galactosidase, which can be referred to as "enzyme donor"; and 2) SHP fused to b-galactosidase -2 polypeptides, which may be referred to as "enzyme receptors". PD-1 antibodies are contacted with cells, and when PD-1 is engaged, SHP-2 is recruited to PD-1. The enzyme acceptor and enzyme donor form a fully active b-galactosidase that can be assayed. This assay can be used to show the activation of PD-1 signaling.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com