Modified membrane type serine protease 1 (mtsp-1) polypeptides and methods of use
A technology of MTSP-1 and serine protease, which is applied in the field of modified membrane-type serine protease 1 (MTSP-1) polypeptide and its application, and can solve problems such as short half-life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0660] The preparation of fusions of therapeutic proteins with other moieties such as PEGylation, conjugation to albumin, targeting moieties such as antibodies and antigen-binding fragments thereof, immunoglobulins, fc fusions, and Fusions of albumin (HSA), XTEN fusion proteins, modification of glycosylation patterns (for a review of various fusion proteins used to improve the pharmacokinetic properties of therapeutic proteins, see Strohl (2015) BioDrugs 29:215 -239). Any of these known modes for improving the pharmacological properties of therapeutic agents can be applied to the modified MTSP-1 polypeptides provided herein.
[0661] Fusion proteins comprising a modified MTSP-1 polypeptide provided herein and one or more other polypeptides are also provided. Pharmaceutical compositions containing such fusion proteins formulated for administration by appropriate routes are provided. Fusion proteins are formed by linking a modified MTSP-1 polypeptide and another polypeptide (s...
Embodiment 1
[0769] Cloning and Expression of Modified MTSP-1 Polypeptides, and Screening for Modified MTSP-1 Polypeptides Cleaving C3 at Target Sites
[0770] A. Cloning and mutagenesis of MTSP-1
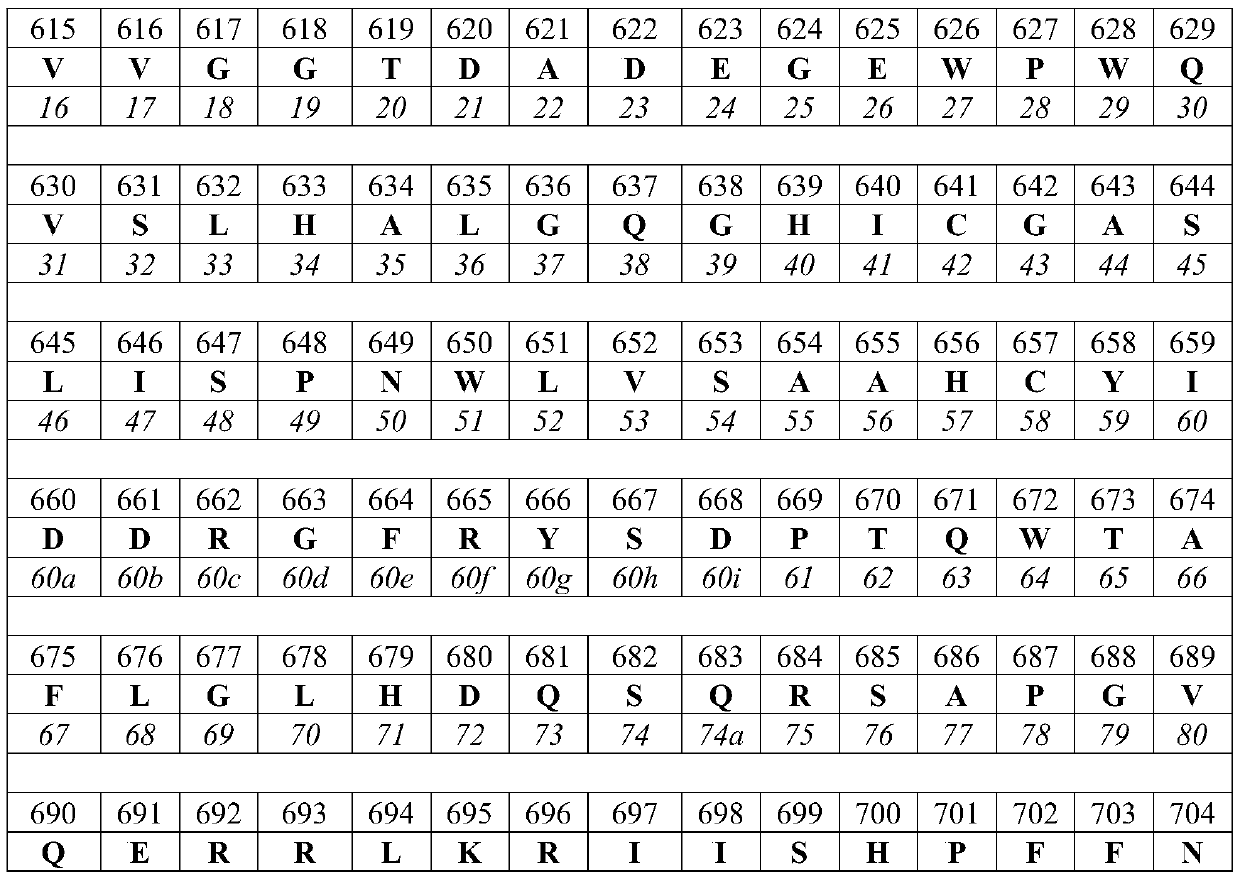
[0771] A nucleic acid encoding amino acids 615-855 having the C122S substitution of the human MTSP-1 polypeptide shown in SEQ ID NO: 1 was prepared. The construct includes a proregion, an activation sequence, and a protease domain, and contains residues 598 to C of the sequence disclosed by Takeuchi et al. (1999) Proc. Natl. Acad. Sci. U.S.A. 96: 11054 and SEQ ID NO: 1 terminal (ie, corresponding to residues 598 to 855 of the amino acid sequence shown in SEQ ID NO: 1).
[0772] Modified MTSP-1 polypeptides were generated by Quikchange site-directed mutagenesis (Stratagene) according to the manufacturer's instructions with specially designed oligonucleotides that served as primers to introduce designed mutations into newly synthesized inside the DNA. Briefly, PCR sample reactions were set up ...
Embodiment 2
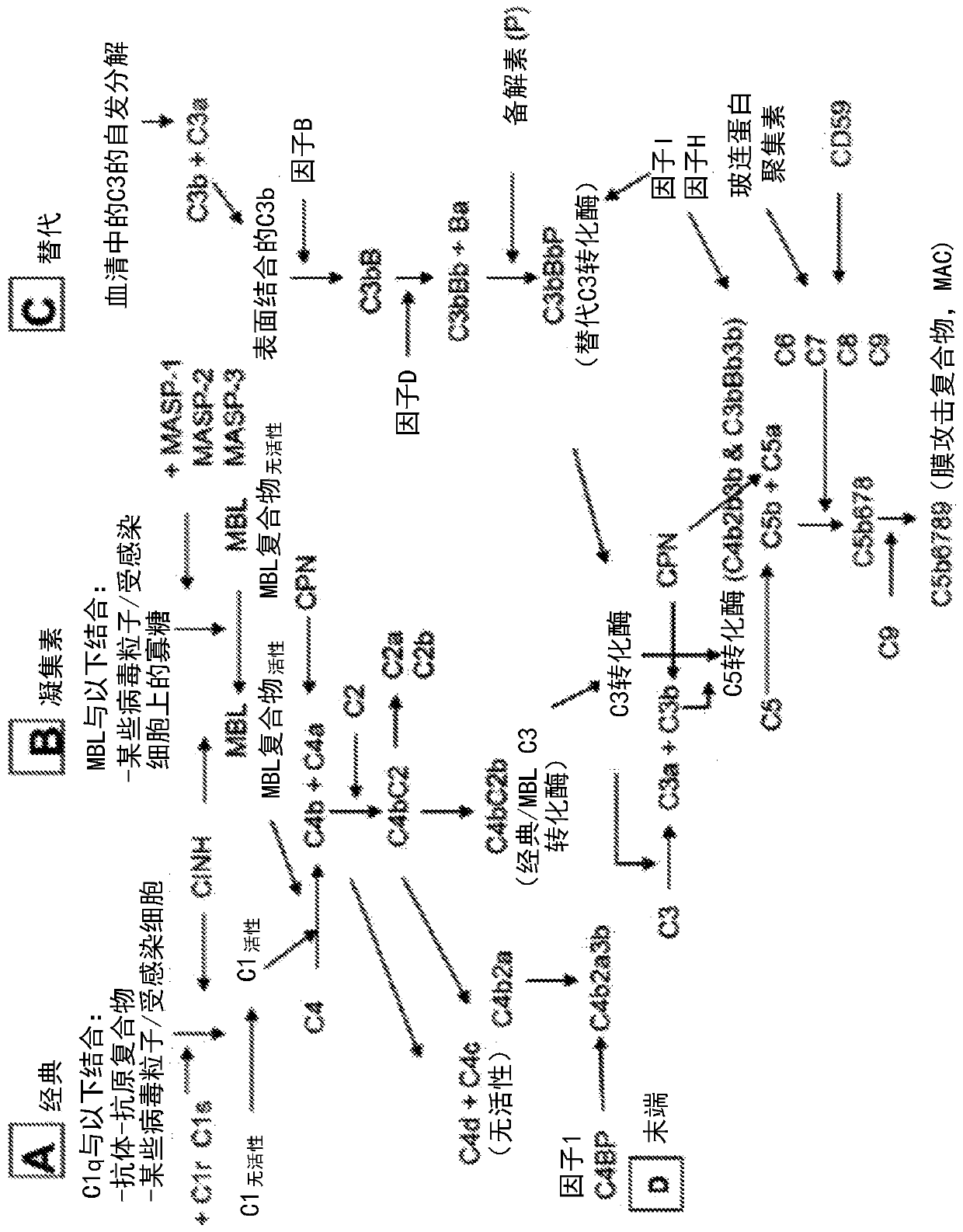
[0820] In vitro cleavage of complement protein C3
[0821] The activity of the modified MTSP-1 polypeptide was determined by cleavage of the substrate complement protein human C3 by measuring the amount of intact human C3 remaining after incubation with various concentrations of protease for 1 hour at 37°C. In this assay, signal is generated in the presence of intact human C3 and lost upon C3 cleavage.
[0822] 2 μM plasma-purified human C3 (hC3; Complement Technologies; Tyler, TX) was incubated with modified MTSP-1 polypeptides (0–250 nM) at 37° C. in the presence of 50 mM Tris, pH 8.0, 50 mM NaCl and 0.01% Tween -20 buffer for 1 hour. The activity of the modified MTSP-1 polypeptide was quenched by adding EGR-CMK (Haematologic Technologies, EGRCK-01) to a final concentration of 10 μM, and the hC3 / modified MTSP-1 polypeptide mixture was allowed to stand at ambient temperature for 30 minute.
[0823] Using Amplified Luminescent Proximity Homogeneous Assay Screen ( Perkin El...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com