Human monoclonal antibody to human tumor necrosis factor
A technology of tumor necrosis factor and cloning antibody, which is applied in the biological field, can solve the problem of patients losing treatment effect, and achieve the effects of strong neutralizing activity, enhanced anti-aggregation, and good specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] Embodiment 1: Obtaining of FCO antibody sequence
[0015] According to the existing literature (Zhu Z, Dimitrov DS, Methods Mol Biol, 2009), a human Fab phage display library was constructed, and human TNFα protein was screened for four rounds based on the library, and by monoclonal phageELISA, the The positive clone was sent for sequencing, and the base sequence of the FCO candidate clone was obtained, and its amino acid sequence was obtained through the amino acid translation software (https: / / web.expasy.org / translate / ), and the light chain amino acid sequence is shown as SEQNo.1 Shown, its heavy chain amino acid sequence is shown in SEQ No.2.
Embodiment 2
[0016] Example 2: Using Tango software to optimize the obtained FCO amino acid sequence
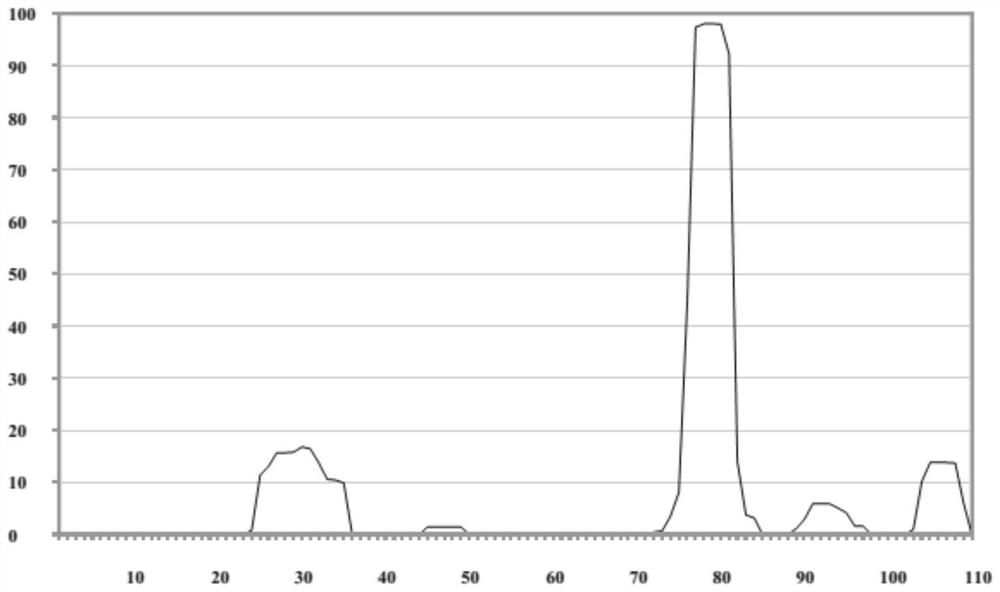
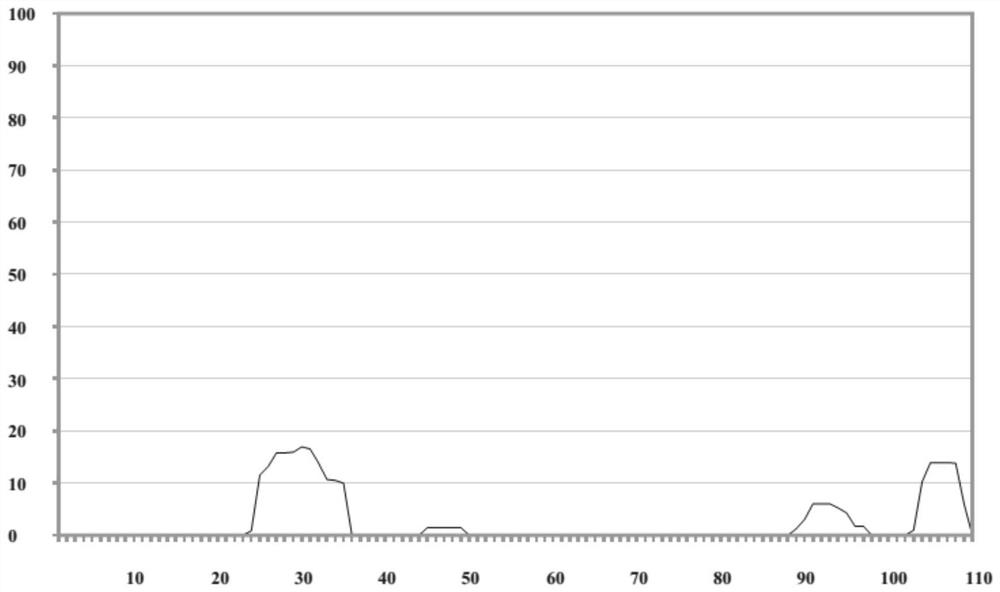
[0017] Use Tango software (http: / / tango.crg.es / ) to predict the aggregation-prone region of the amino acid sequence of the FCO candidate clone, and find that there is a strong aggregation-prone region, such as Picture 1-1 , so point mutation is carried out in this region to obtain FC9 antibody, the amino acid sequence of its light chain is shown in SEQ No.1, the amino acid sequence of its heavy chain is shown in SEQ No.3, the corresponding base sequence, and the light chain is shown in SEQ No. 4, the heavy chain is shown in SEQ No.5. The mutated amino acid sequence was re-predicted by Tango software, and it was found that the aggregation-prone region was significantly improved, as shown in Figure 1-2 , 1-3.
Embodiment 3
[0018] Example 3: 293F cells express FC9 antibody and detect it by SDS-PAGE
[0019] The mutated base sequence was cloned into the dual promoter vector pVitro2-neo-mcs (InvivoGen), and the 293F cells were transfected with PEI to express the protein. After 5-7 days of expression, the culture supernatant of the 293F cells was treated with ProteinA (GE) was purified, and after the purified protein was concentrated and exchanged, the FC9 antibody dissolved in PBS buffer was obtained. After SDS-PAGE electrophoresis detection, it was found that it presented two bands of light chain and heavy chain, such as figure 2 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com