A method for preparing gem-diboron compounds by selective 1,1-diboration of alkenes
A compound and selective technology, applied in the field of selective 1,1-diboronation of olefins to prepare geminal diboron compounds, can solve the problems of reduced atom economy, limited substrate source, poor compatibility of functional groups, etc. Product separation and purification, improved atom economy, mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
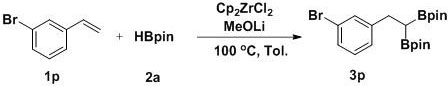
[0022] Example 1 A method for the selective 1,1-diboration of olefins to prepare gem-diboron compounds:
[0023]
[0024] Add Cp to the reaction tube sequentially 2 ZrCl 2 (0.01 mmol, 2.9 mg), MeOLi (0.2 mmol, 7.6 mg), toluene (1 mL), pinacol borane 2a (0.6 mmol, 87 μL), styrene 1a (0.2 mmol, 23 μL), in nitrogen (1atm ) atmosphere at 100 °C for 8 h with stirring. GC-MS detection yield was 88%.
Embodiment 2
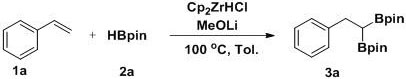
[0025] Example 2 A method for the selective 1,1-diboration of olefins to prepare gem-diboron compounds:
[0026]
[0027] Add Cp to the reaction tube sequentially 2 ZrCl 2 (0.01 mmol, 2.9 mg), MeOLi (0.2 mmol, 7.6 mg), toluene (1 mL), pinacol borane 2a (0.6 mmol, 87 μL), styrene 1a (0.2 mmol, 23 μL), in nitrogen (1atm ) atmosphere at 130 °C for 2 h with stirring. GC-MS detection yield was 92%.
Embodiment 3
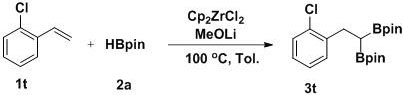
[0028] Example 3 A method for the selective 1,1-diboration of olefins to prepare gem-diboron compounds:
[0029]
[0030] Add Cp to the reaction tube sequentially 2 ZrCl 2 (0.01 mmol, 2.9 mg), MeOLi (0.2 mmol, 7.6 mg), toluene (1 mL), pinacol borane 2a (0.6 mmol, 87 μL), styrene 1a (0.2 mmol, 23 μL), in nitrogen (1atm ) atmosphere at 80 °C for 16 h with stirring. GC-MS detection yield was 81%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com