Synthesis method of (3-cyclopropylpyridin-2-yl) methylamine
A technology for the synthesis of cyclopropylpyridine and its method, which is applied in the field of synthesis of (3-cyclopropylpyridin-2-yl)methylamine, achieving the effects of high yield, simple operation and short route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
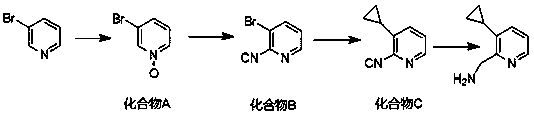
[0025] A kind of synthetic method of (3-cyclopropylpyridin-2-yl) methylamine, this synthetic method comprises the steps:
[0026] (1) Dissolving 3-bromopyridine in an organic solvent, adding an oxidant at -10 to 0°C, and performing an oxidation reaction to obtain Compound A;
[0027] (2) Dissolve compound A in acetonitrile, under nitrogen protection, and carry out cyanation reaction with trimethylsilyl cyanide and triethylamine at 70-80°C to obtain compound B;
[0028] (3) Dissolve compound B in toluene, sequentially add cyclopropylboronic acid, potassium phosphate, tricyclohexylphosphine, water, and palladium acetate under nitrogen protection, and carry out coupling reaction at 100-105°C to obtain compound C;
[0029] (4) Dissolve compound C in methanol, add Raney nickel and ammonia water in a hydrogen atmosphere, and perform a reduction reaction to obtain (3-cyclopropylpyridin-2-yl)methylamine.
[0030] In the step (1), the organic solvent is dichloromethane, the oxidizing ...
Embodiment
[0035] Compound A: Synthesis of 3-bromopyridine monoxide
[0036]
[0037] Dissolve 20g of 3-bromopyridine in 300mL of organic solvent dichloromethane, add 48g of oxidant m-chloroperoxybenzoic acid at -10~0°C, stir, and carry out oxidation reaction for 3.5h, then add saturated carbonic acid under ice bath Sodium hydrogen aqueous solution 120mL, stirred for 30min, dichloromethane and methanol were mixed at a volume ratio of 8:1, extracted 7 times, and the organic phase was dried with anhydrous sodium sulfate, concentrated, and column chromatographed to obtain 20g of 3-bromo Pyridine monoxide, the yield is 90.8%.
[0038] Compound B: Synthesis of 3-bromopyridine-2-carbonitrile
[0039]
[0040] Dissolve 20 g of the prepared 3-bromopyridine monoxide in 300 mL of acetonitrile, under nitrogen protection, and 35 g of trimethylsilyl cyanide and 24 g of triethylamine at 70 to 80 ° C, reflux overnight to carry out cyanation reaction, After cooling, the reaction solution was pou...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap