Electrolyte and lithium secondary battery comprising same
A lithium secondary battery and electrolyte technology, applied in non-aqueous electrolyte batteries, secondary batteries, lithium batteries, etc., can solve problems such as collapse, reduced battery life, and reduced battery charging/discharging efficiency, and achieve the effect of preventing growth
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
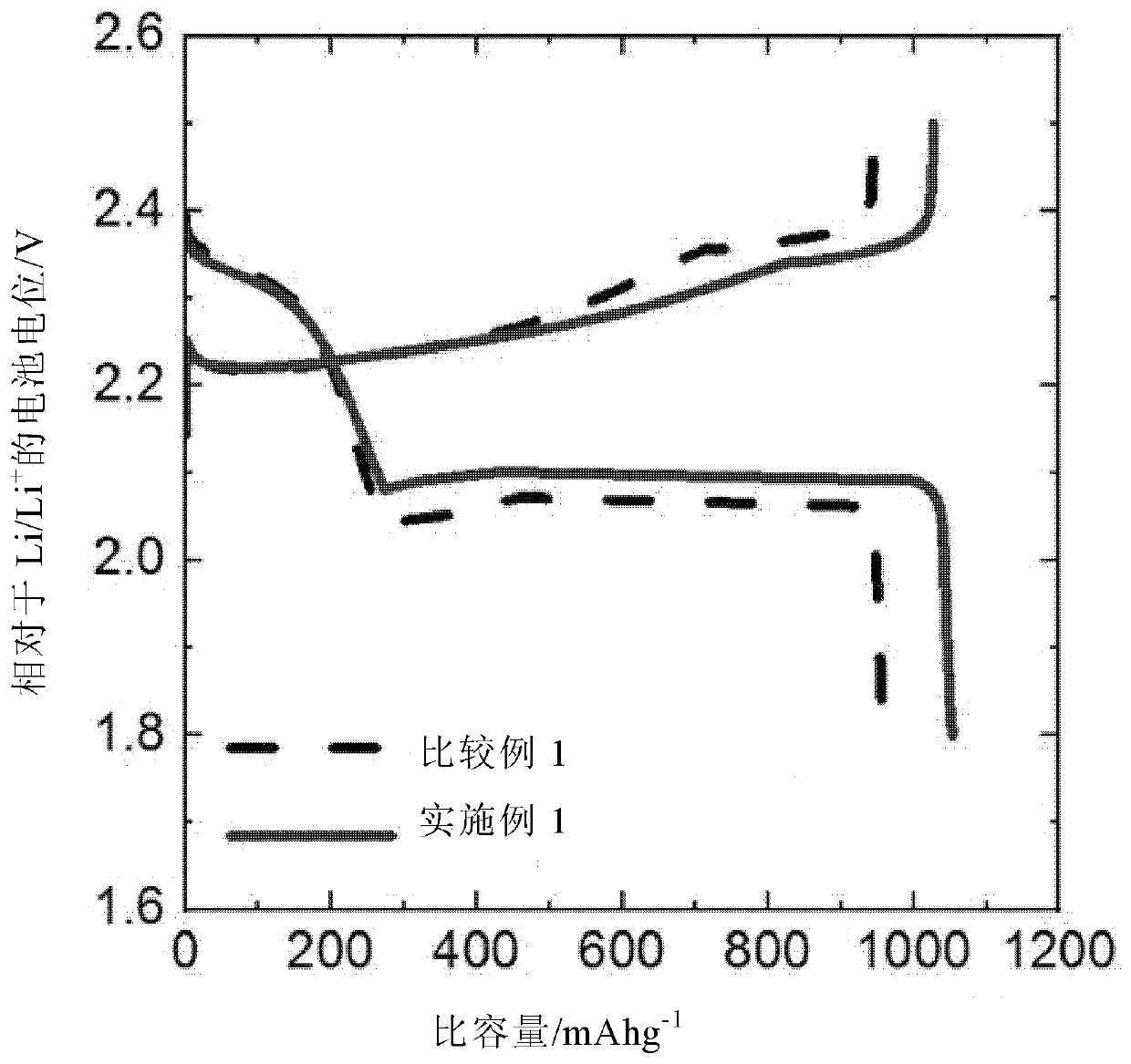
[0105] Embodiment 1: the preparation of lithium-sulfur battery
[0106] The lithium metal negative electrode and the S / C positive electrode were arranged to face each other with a polyethylene (PE) separator placed in between, and then 70 μl of electrolyte was injected to prepare a lithium-sulfur battery in the form of a button cell.
[0107] As the electrolyte, an electrolytic solution (E1) containing 0.1% by weight of an additive was used, and the additive was polyethylene glycol methyl ether thiol (mPEG-SH; molecular weight: 800). Electrolyte (E1) uses DOL / DME (1:1, v / v) as solvent and contains 1M LiTFSI and 3% by weight LiNO 3 electrolyte (DOL: dioxolane; DME: dimethoxyethane).
Embodiment 2
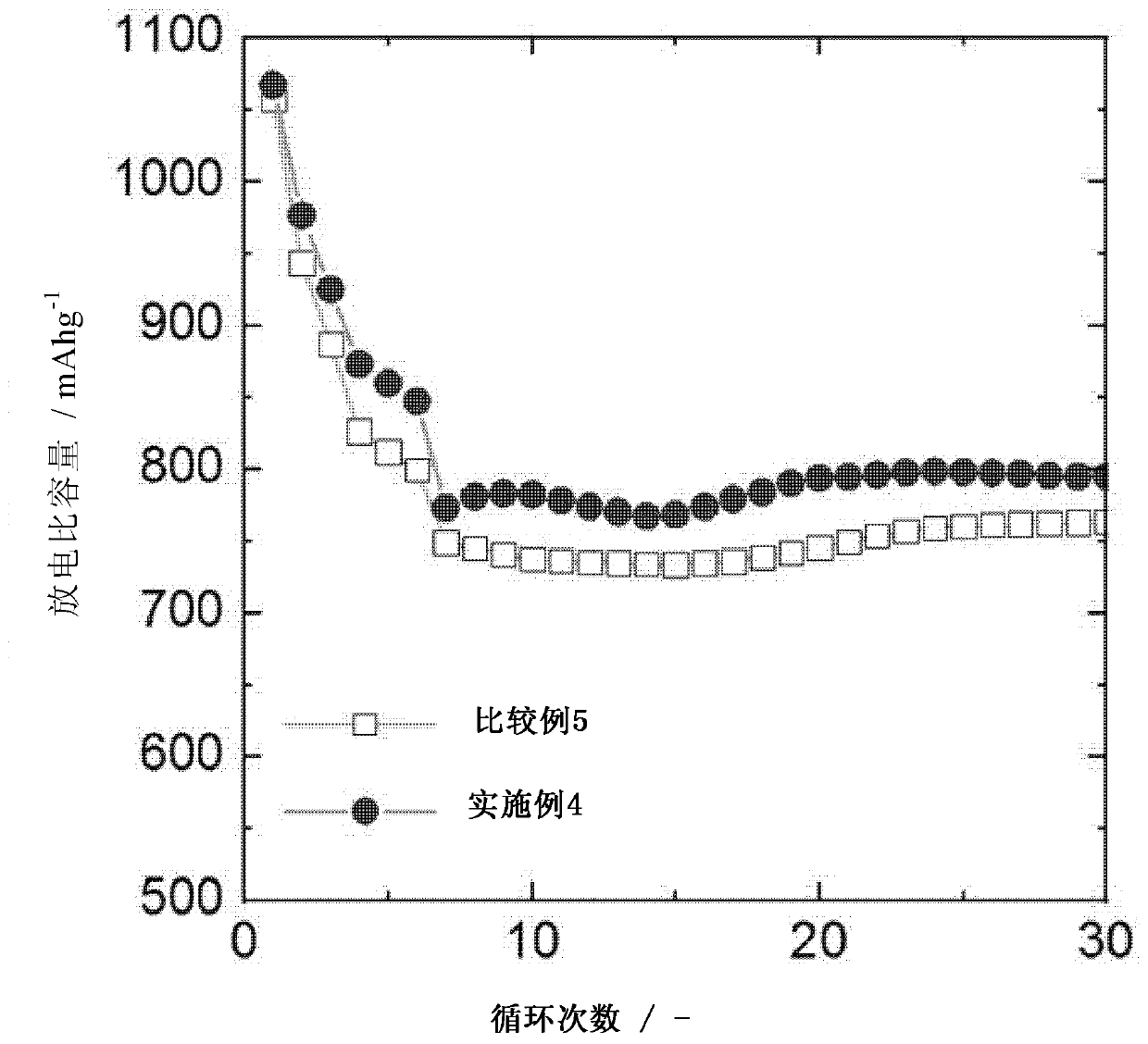
[0110] Embodiment 2: Preparation of lithium-lithium symmetric battery
[0111] A lithium metal negative electrode and a lithium metal positive electrode were arranged to face each other with a polyethylene (PE) separator placed therebetween, and then 100 μl of electrolyte was injected to prepare a lithium-lithium battery in the form of a button cell.
[0112] As the electrolyte, an electrolytic solution (E2) containing 0.1% by weight of an additive was used, and the additive was polyethylene glycol methyl ether thiol (mPEG-SH; molecular weight: 800). The electrolytic solution (E2) used DOL / DME (1:1, v / v) as a solvent and was an electrolytic solution containing 1M LiTFSI (DOL: dioxolane; DME: dimethoxyethane).
Embodiment 3
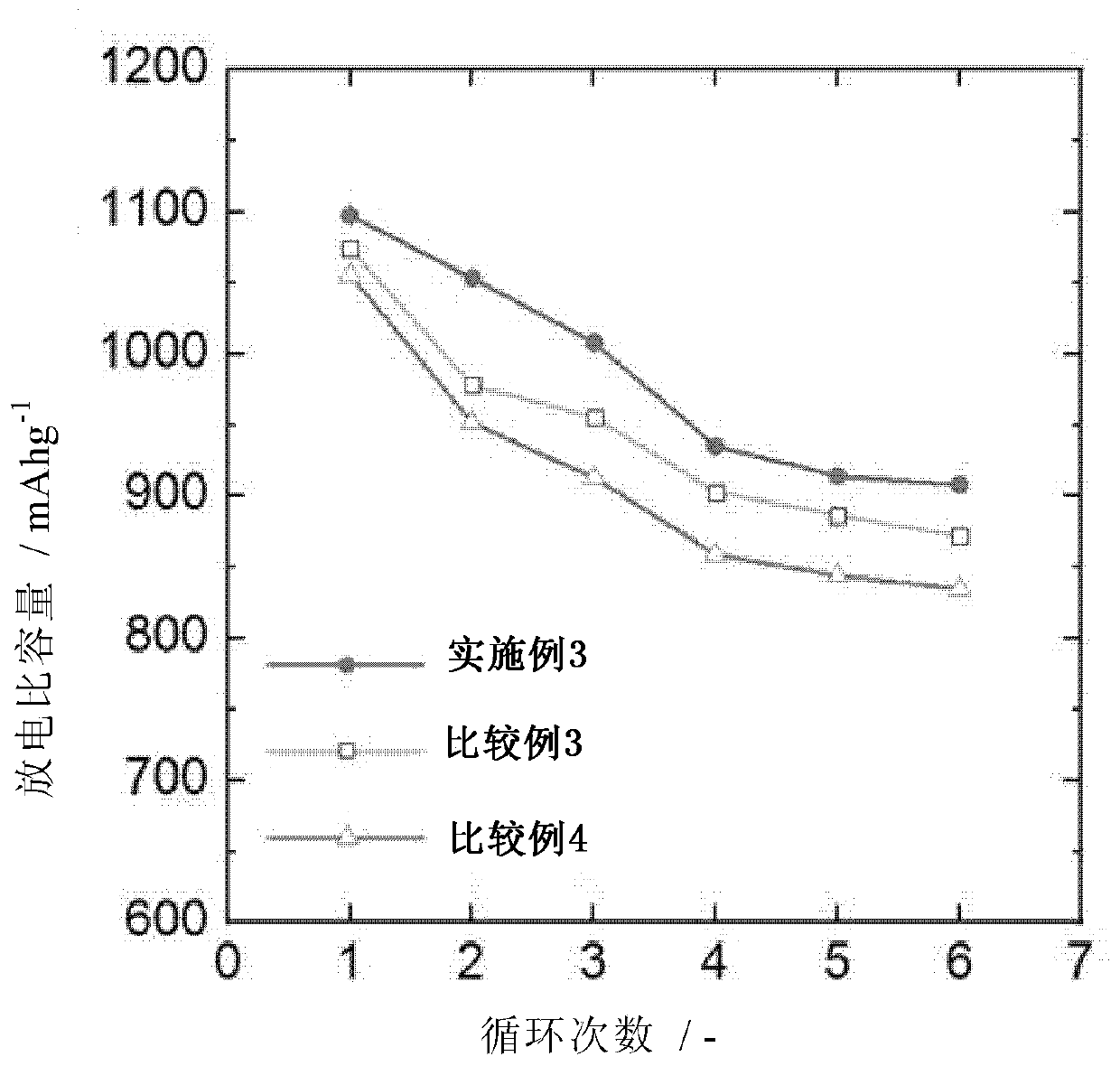
[0115] Embodiment 3: the preparation of lithium-sulfur battery
[0116] The lithium metal negative electrode and the S / C positive electrode were arranged to face each other with a polyethylene (PE) separator placed therebetween, and then 70 μl of electrolyte was injected to prepare a lithium-sulfur battery in the form of a button cell.
[0117] As the electrolyte, an electrolytic solution (E1) containing 0.1% by weight of an additive was used, and the additive was polyethylene glycol methyl ether thiol (mPEG-SH; molecular weight: 2000).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com