Macrobrachium rosenbergii thymosin β4 gene, protein, preparation method and application thereof
A technology of Macrobrachium rosenbergii and thymosin, which is applied in the preparation methods of peptides, thymosin, peptide/protein components, etc., to achieve the effect of obvious antibacterial activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
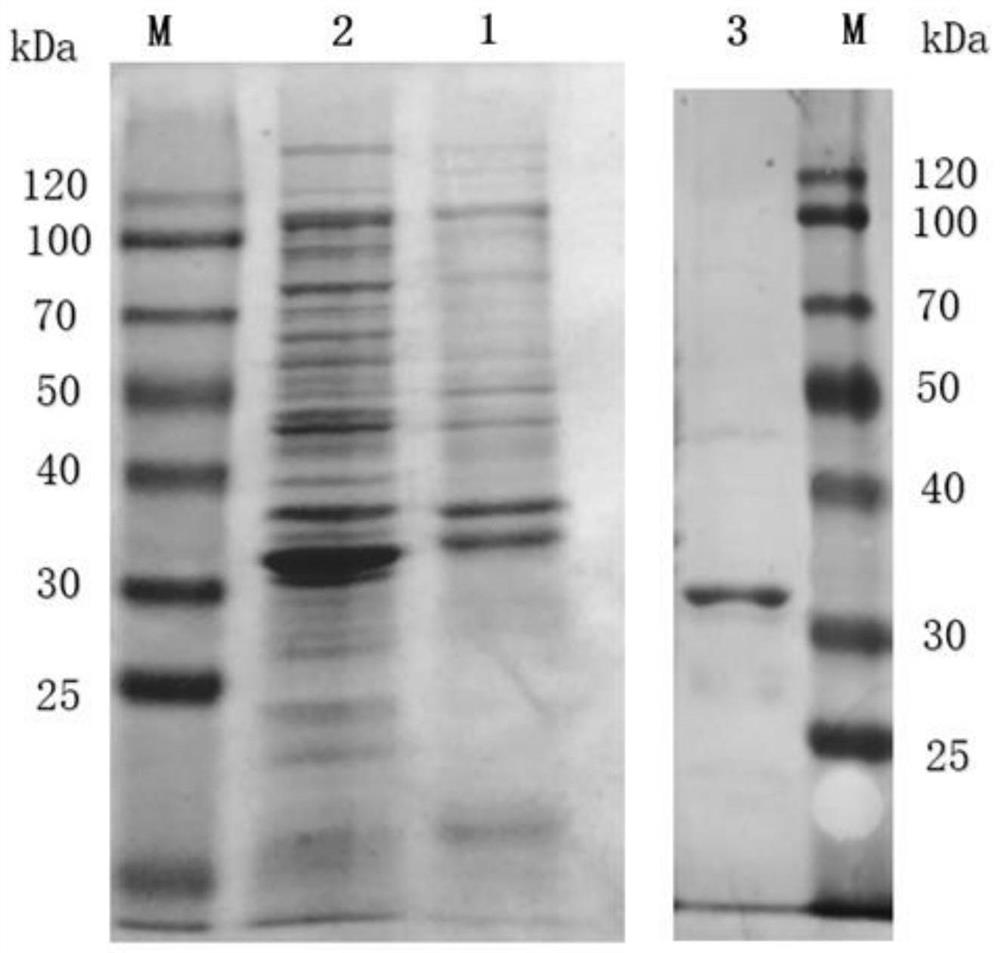
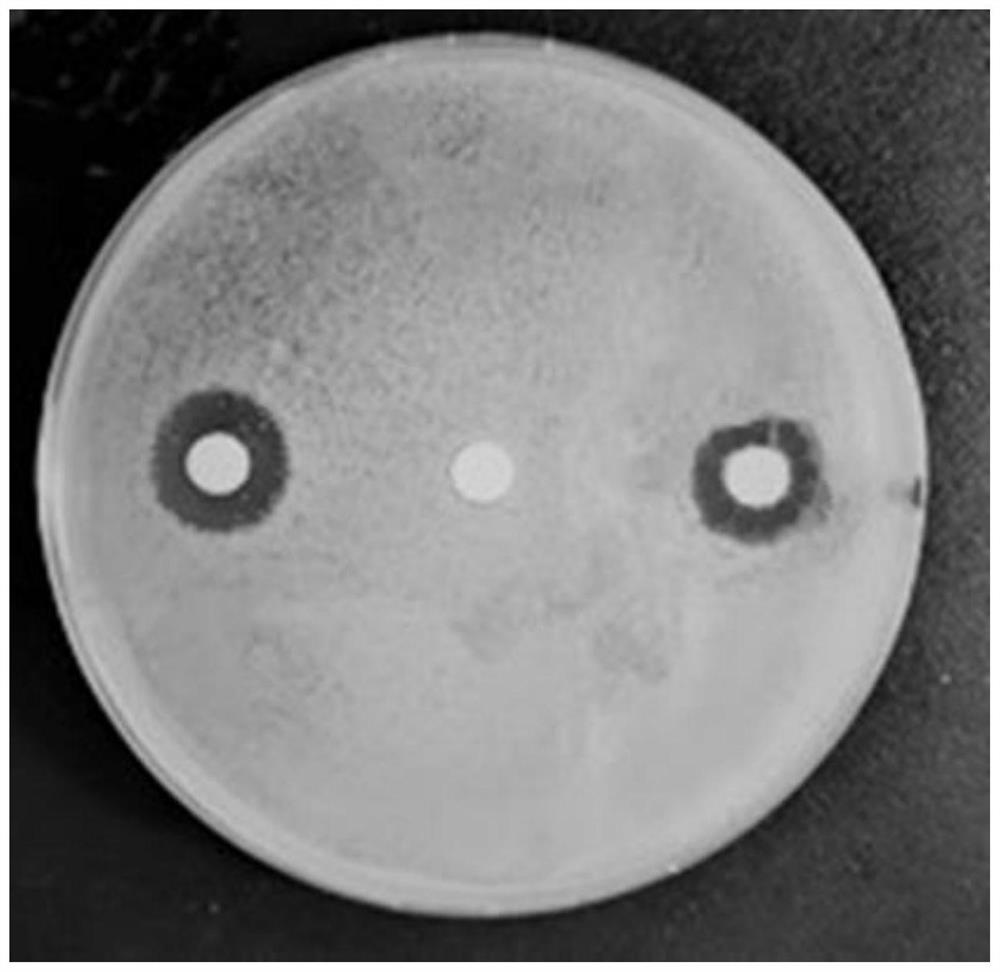
Image
Examples
Embodiment 1
[0036] 1. Transformation of MrThyβ4-pET30A recombinant plasmid:
[0037] Take competent cells E.coli BL21 and put them in ice until they melt. Add the recombinant plasmid to the competent cells, mix the contents gently, and let stand in an ice bath for 3 minutes. Place the centrifuge tube in a 42°C water bath for 90 seconds, quickly transfer to an ice bath, and cool for 2-3 minutes without shaking. Add 900 μL of sterile LB medium (without antibiotics) to each centrifuge tube, mix well, and place on a shaker at 37°C for 45 minutes (15 rpm / min) to revive the bacteria. Place the recovered bacterial solution in a centrifuge at 1000rpm, centrifuge for 3min, discard 850μL supernatant, mix well, draw competent cells and add them to the LB solid medium containing the corresponding antibiotics, use a sterile elbow glass rod Gently spread the cells evenly, place at room temperature until the liquid is absorbed, invert the plate, and incubate at 37°C for 12-16h.
[0038] 2. Induced ex...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com