Elarylamine derivative, hole transport material comprising the same, and organic electroluminescence device using the same
A derivative, arylamine technology, applied in the field of arylamine derivatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
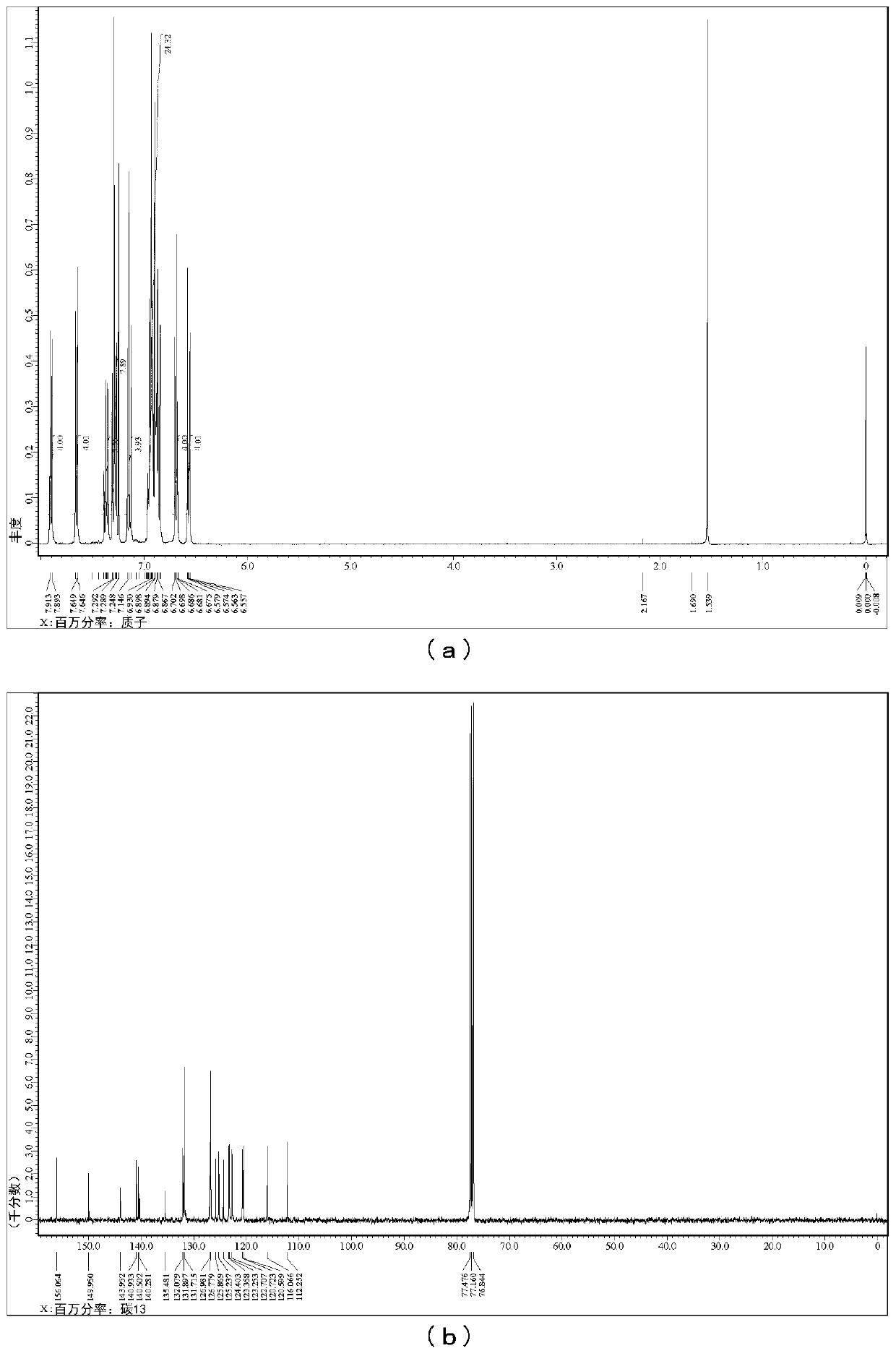
[0080] [Example 1] Synthesis of T4DBFHPB
[0081] (i) 4DBFNCPh 2 Synthesis
[0082]
[0083] Add 2.47g (10mmol) of 4-BrDBF, 1.68ml (10mmol) of benzophenone imine, 2.5mg (2.6mmol) of tertiary BuONa, and 50ml of dry toluene to a 25ml three-necked flask, and carry out N for 1 hour. 2 bubbling. After that, add Pd 2 (dba) 3 370 mg (0.4 mmol) and ± BINAP 560 mg (0.9 mmol), stirred at 90°C under nitrogen. After confirming the consumption of the raw material by TLC, it was cooled to room temperature, filtered through a glass filter filled with silica gel, concentrated, and dried under reduced pressure to obtain the target product.
[0084] Yield 3.39g, yield 98%
[0085] MS: [M + ]=348.1m / z
[0086] (ii) 4DBFNH 2 Synthesis
[0087]
[0088] Add 4DBFNCPH to a 100ml eggplant flask 2 3.39 mg (9.8 mmol), 50 ml of THF and 50 ml of 2M HClaq were stirred under reflux for 5 hours. The disappearance of the raw material and the production of the target product were confirmed...
Embodiment 2
[0103] [Example 2] Synthesis of TDBFBP
[0104]
[0105] In a 30ml four-necked flask, add 1.048g (3.0mmol) of B4DBFNH synthesized in Example 1, 468mg (1.5mmol) of 3,3'-dibromobiphenyl, 750mg (7.8mmol) of tertiary BuONa and 30ml of dry toluene to carry out 2 hours 2 bubbling. After that, add Pd 2 (dba) 3 137mg (0.15mmol) and t-Bu 3 P + BF 4 - 87 mg (0.3 mmol), stirred under reflux for 22 hours under nitrogen flow. The disappearance of the raw material was confirmed by TLC, and it returned to normal temperature. Thereafter, extraction was performed three times with 100 ml of toluene, and the organic layer was dried over anhydrous magnesium sulfate, filtered, and concentrated. Purification was carried out by silica column chromatography (developing solvent: toluene / hexane=1 / 1) to obtain 1.082 g of the target product at a yield of 78%. Target identification through 1 H-NMR, 13 C-NMR and MS to carry out.
[0106] 1 H-NMR (400MHz, CDCl 3 )δ=7.92(dd, J=8.0, 1.1Hz, ...
Embodiment 3
[0109] [Example 3] Synthesis of TDBFP
[0110]
[0111] Add B4DBFNH 2.795g (8.0mmol), 1,3-dibromobenzene 944mg (4.0mmol), tertiary BuONa 2.000g (20.8mmol) and dry toluene 80ml in a 100ml four-necked flask, and carry out 2 hours N 2 bubbling. After that, add Pd 2 (dba) 3 366mg (0.40mmol) and t-Bu 3 P + BF 4 - 232mg (0.8mmol), stirred under reflux for 22 hours under nitrogen flow. After confirming disappearance of the raw material by TLC, Celite filtration was performed, and the filtrate was concentrated. Purified by silica gel chromatography (developing solvent: toluene), concentrated, and washed with methanol to obtain 2.866 g of the target product in a yield of 93%. Target identification through 1 H-NMR, 13 C-NMR and MS to carry out.
[0112] 1 H-NMR (600MHz, CDCl 3 )δ=7.90(d, J=7.6Hz, 4H), 7.60-7.56(m, 4H), 7.41-7.35(m, 8H), 7.34-7.29(m, 4H), 7.14(d, J=6.9Hz , 4H), 7.06(t, J=7.9Hz, 1H), 6.84(t, J=7.6Hz, 4H), 6.71(t, J=2.4Hz, 1H), 6.60(dd, J=7.9, 2.4Hz ,2H)...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap