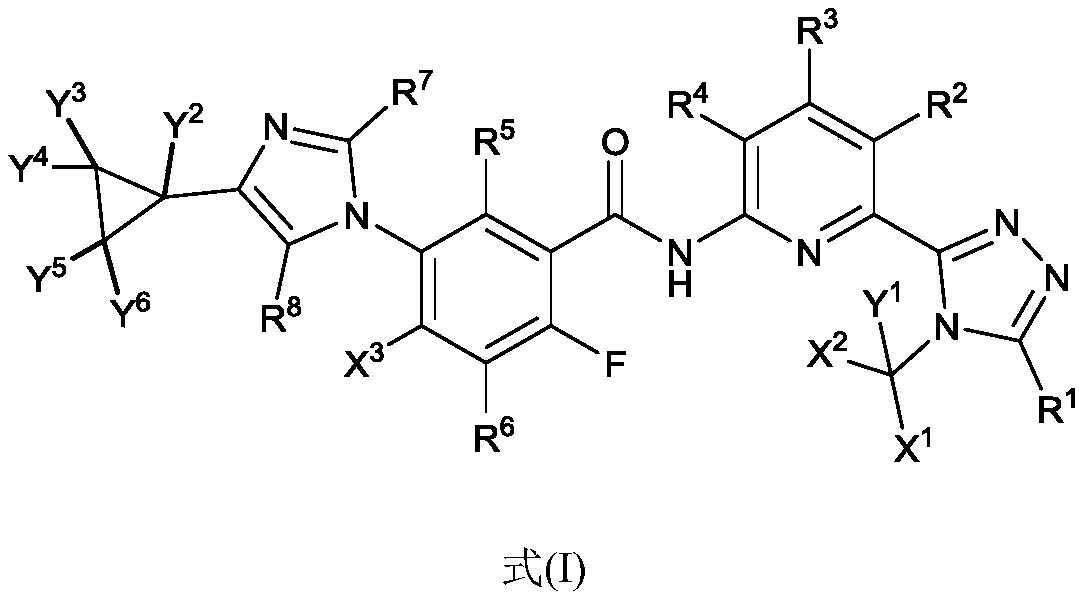
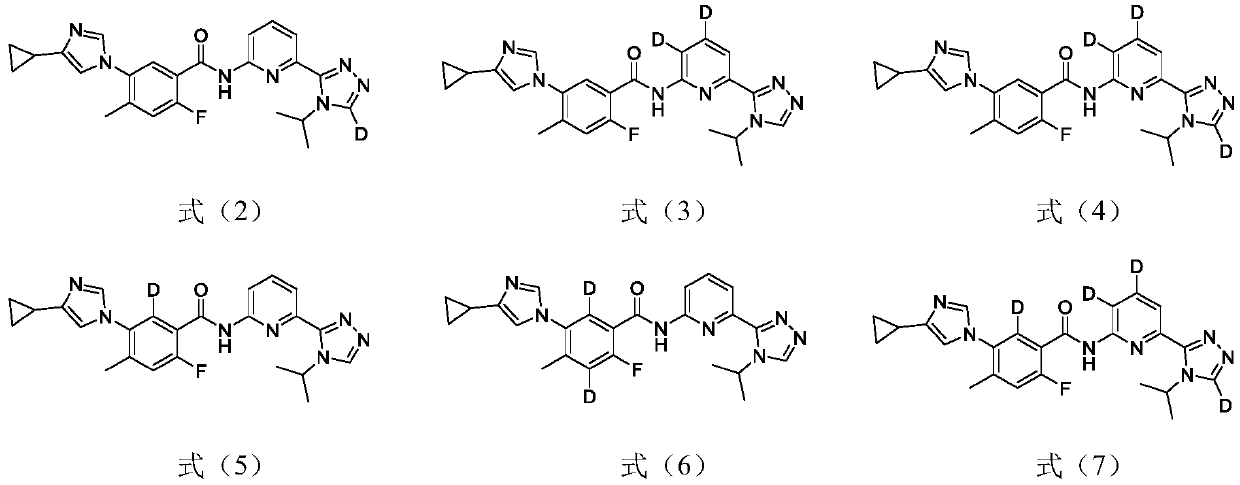
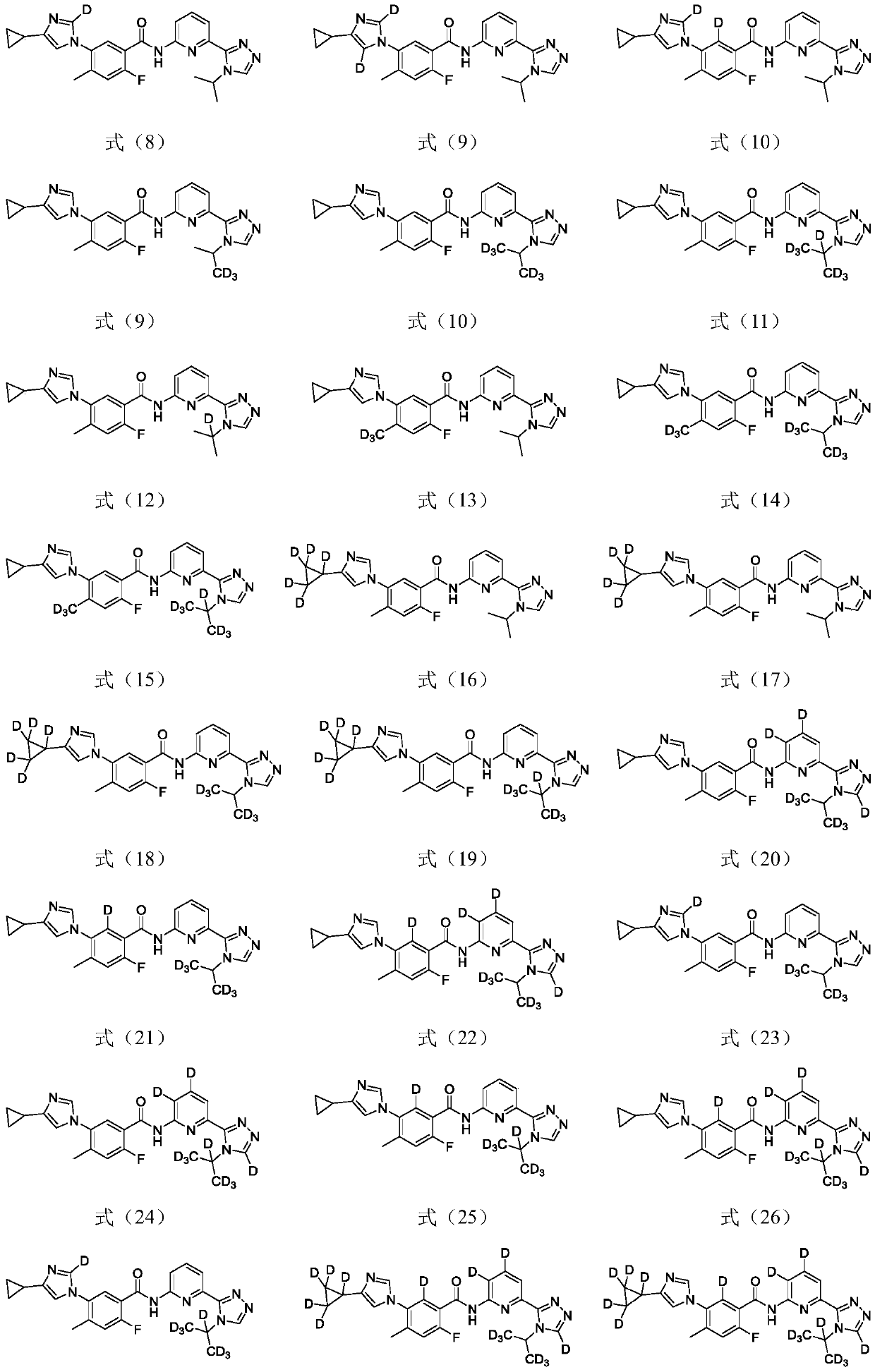
1,2,4-triazole compound
一种化合物、三氮唑的技术,应用在医药领域,能够解决代谢清除率降低等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0105] Example 1 5-(4-cyclopropyl-1H-imidazolyl-1-yl)-N-(6-(4-isopropyl-4H-1,2,4-triazole-3- Base-5-d)pyridin-2-yl-3,4-d 2 ) - Preparation of 2-fluoro-4-methylbenzamide (compound T-1).
[0106]
[0107] Concrete synthetic steps are as follows:
[0108]
[0109] Step 1 Synthesis of compound 2.
[0110] Add compound 1 (5.0g, 32.86mmol) and methanol (60mL) to a 100mL single-necked flask equipped with magnetic stirring in turn, stir to dissolve, and slowly add hydrazine hydrate (3.29g, 65.72mmol) dropwise. The liquid was heated under reflux for 3 hours, then cooled to room temperature, a large amount of white solid was precipitated, filtered, the filter cake was washed with cold methanol, and dried to obtain 3.5 g of the white solid, with a yield of 70%. LC-MS(APCI):m / z=153.2(M+1) + . 1 H NMR (DMSO-d 6 ,300MHz)(δ / ppm):9.14(s,1H),7.51(t,J=5.7Hz,1H),7.11(d,J=5.7Hz,1H),6.61(d,J=6.0Hz,1H ),6.08(s,2H),4.48(s,2H).
[0111] Step 2 Synthesis of compound 3.
[0112] Add com...
Embodiment 2
[0125] Example 2 5-(4-cyclopropyl-1H-imidazolyl-1-yl)-N-(6-(4-isopropyl-4H-1,2,4-triazole-3- yl)pyridin-2-yl)-2-fluoro-4-methylbenzamide-6-d (compound T-2).
[0126]
[0127] Concrete synthetic steps are as follows:
[0128]
[0129] Step 1 Synthesis of Compound 12.
[0130] Add compound 6 (2.0g, 9.8mmol) and heavy water (10mL) to a 20mL microwave tube equipped with magnetic stirring, and slowly add a heavy aqueous solution of DCl (0.817mL, 9.8mmol, 12M) under stirring. Lower the temperature to 160°C and react for 1.5 hours. Cool to room temperature, saturated NaHCO 3 Adjust the pH to 10, extract with dichloromethane (20mLx3), combine the organic phases, dry over anhydrous sodium sulfate, filter, and concentrate to obtain 1.59g of a brown solid, with a yield of 79.1%. LC-MS(APCI):m / z=205.1(M+1) + . 1 H NMR (DMSO-d 6 ,300MHz)(δ / ppm):6.93(d,J=7.2Hz,1H),4.94(s,2H),1.99(s,3H).
[0131] Step 2 Synthesis of compound 13.
[0132] Add compound 12 (1.59g, 7.75mmol) an...
Embodiment 3
[0141] Example 3 5-(4-cyclopropyl-1H-imidazolyl-1-yl)-N-(6-(4-(prop-2-yl-1,1,1,3,3,3-d 6 )- Preparation of 4H-1,2,4-triazol-3-yl)pyridin-2-yl)-2-fluoro-4-methylbenzamide (Compound T-3).
[0142]
[0143] Concrete synthetic steps are as follows:
[0144]
[0145] Step 1 Synthesis of Compound 18.
[0146] Ammonium acetate (6.32 g, 78 mmol) and MeOD (50 mL) were added to a 50 mL one-necked flask equipped with magnetic stirring, N 2 The temperature was raised to reflux under atmosphere for 3 hours. Concentrate to dryness under reduced pressure, then add MeOD (10mL), add acetone-d under stirring 6 (1.0g, 15.6mmol) and NaBH 3 CN (980mg, 15.6mmol), N 2 The reaction was stirred overnight at room temperature under atmosphere. Add water (10mL) to quench the reaction, adjust the pH to 2 with 6M hydrochloric acid, extract with ethyl acetate (20mLx2), adjust the pH to 12 with 6M NaOH in the aqueous phase, extract with dichloromethane (20mLx3), combine the organic phases, an...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap