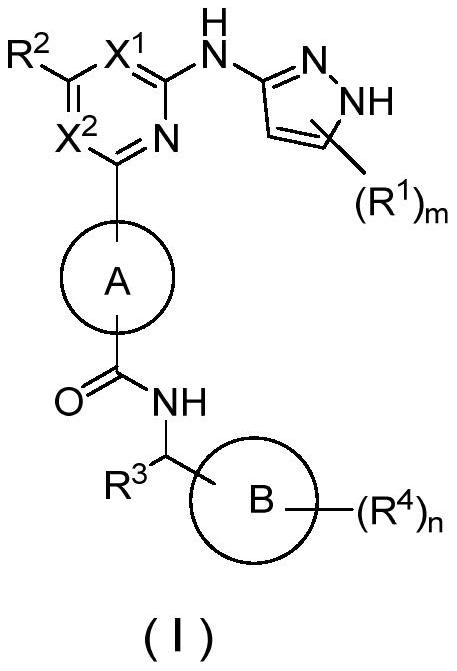
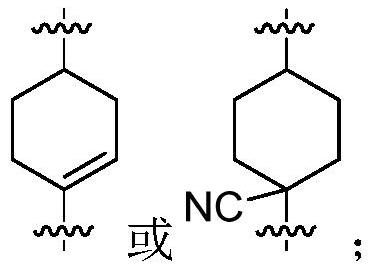
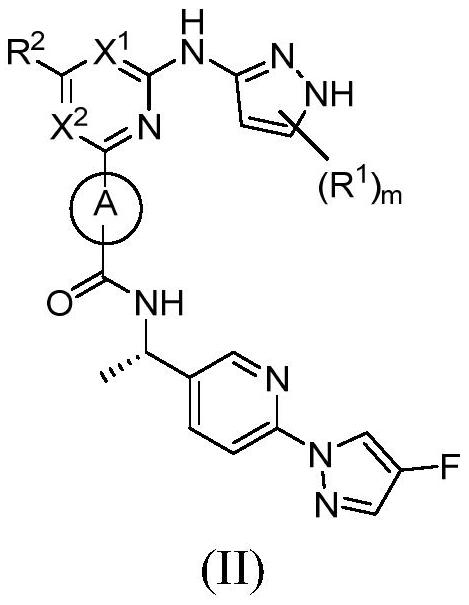
Pyrazole derivative as well as preparation method and medical application thereof
A compound, selected technology, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0121] (S)-1-cyano-N-(1-(6-(4-fluoro-1H-pyrazol-1-yl)pyridin-3-yl)ethyl)-4-(4-methyl-6-((5 -methyl-1H-pyrazol-3-yl)amino)pyrimidin-2-yl)cyclohexane-1-carboxamide 1
[0122] (S)-1-cyano-N-(1-(6-(4-fluoro-1H-pyrazol-1-yl)pyridin-3-yl)ethyl)-4-(4-methyl-6 -((5-Methyl-1H-pyrazol-3-yl)amino)pyrimidin-2-yl)cyclohexane-1-carboxamide 1
[0123]
[0124] first step
[0125] tert-butyl
[0126] 3-((tert-butoxycarbonyl)(2-chloro-6-methylpyrimidin-4-yl)amino)-5-methyl-1H-pyrazole-1-carboxy late 1b
[0127] 3-((tert-butoxycarbonyl)(2-chloro-6-methylpyrimidin-4-yl)amino)-5-methyl-1H-pyrazole-1-carboxylic acid tert-butyl ester 1b
[0128] Dissolve 2-chloro-6-methyl-N-(5-methyl-1H-pyrazol-3-yl)pyrimidin-4-amine 1a (2.7g, 12.0mmol) in 24mL dichloromethane, add di Di-tert-butyl carbonate (5.9g, 27.2mmol), triethylamine (4.2mL, 30mmol), and 4-dimethylaminopyridine (147mg, 1.3mmol) were added at 0°C, and reacted overnight at room temperature. TLC detected that the reaction was complete, ...
Embodiment 2
[0189] N-((S)-1-(6-(4-fluoro-1H-pyrazol-1-yl)pyridin-3-yl)ethyl)-4-(4-methyl-6-((5-methyl-1H -pyrazol-3-yl)amino)pyrimidin-2-yl)cyclohex-1-ene-1-carboxamide 2
[0190] N-((S)-1-(6-(4-fluoro-1H-pyrazol-1-yl)pyridin-3-yl)ethyl)-4-(4-methyl-6-((5- Methyl-1H-pyrazol-3-yl)amino)pyrimidin-2-yl)cyclohex-1-ene-1-carboxamide 2
[0191]
[0192] first step
[0193] tert-butyl
[0194] 3-((tert-butoxycarbonyl)(6-methyl-2-(4-oxocyclohexyl)pyrimidin-4-yl)amino)-5-methyl-1H-pyrazol e-1-carboxylate 2a
[0195] 3-((tert-butoxycarbonyl)(6-methyl-2-(4-oxocyclohexyl)pyrimidin-4-yl)amino)-5-methyl-1H-pyrazole-1-carboxylic acid tert butyl ester 2a
[0196] 4-(4-methyl-6-((5-methyl-1H-pyrazol-3-yl)amino)pyrimidin-2-yl)cyclohexane-1-one 1g (6.0g, 21.0mmol) Dissolve in 42mL of dichloromethane, add di-tert-butyl dicarbonate (10.3g, 47.3mmol), triethylamine (7.3mL, 52.5 mmol), add 4-dimethylaminopyridine (256mg, 2.1mmol) at 0°C , reacted at room temperature for 16 hours. TLC detected that th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com