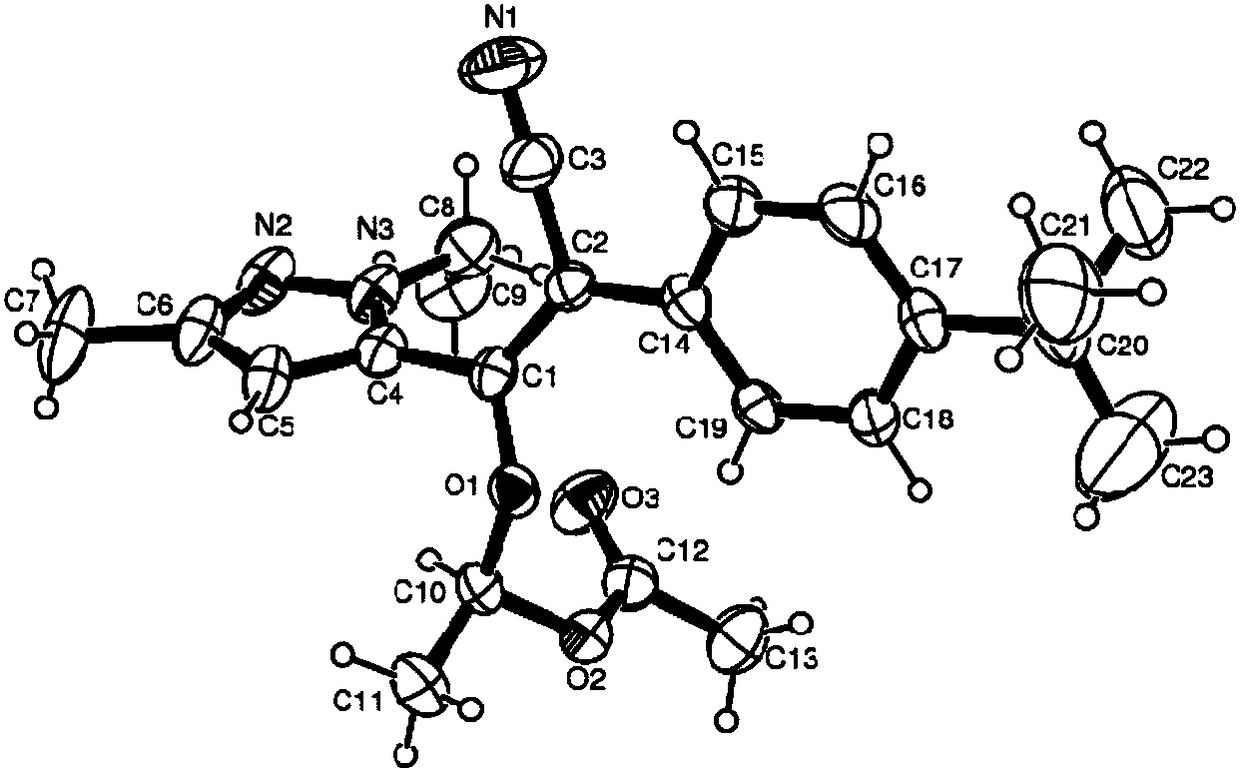
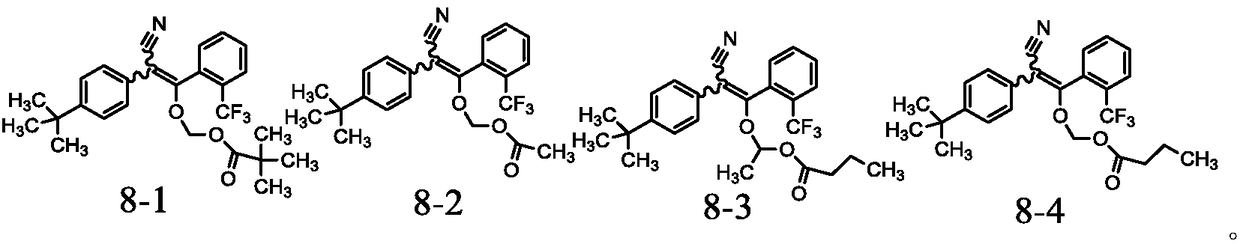
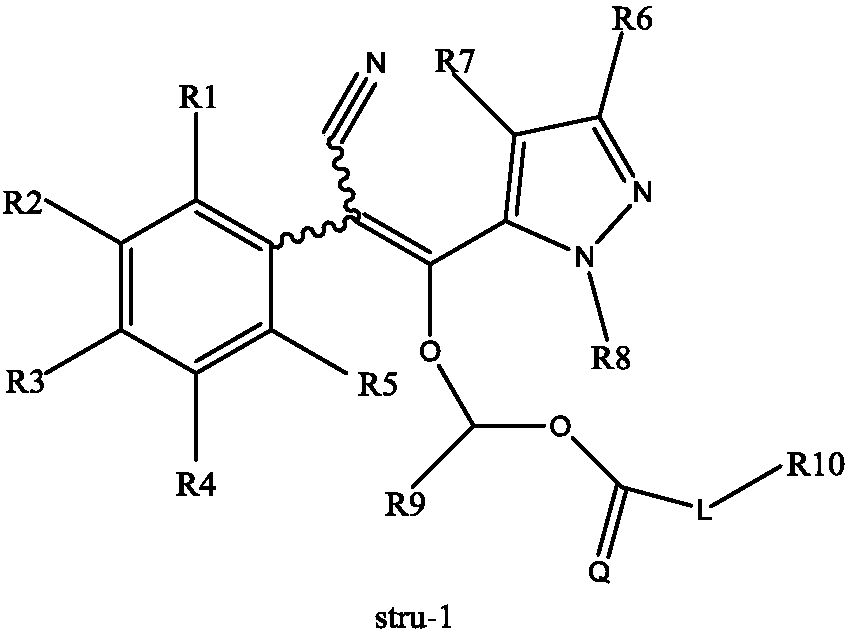
Pyrazol derivative and preparation method and application thereof
A technology of pyrazole derivatives and C1-C20, which is applied in the field of acaricide and agricultural insecticide, and can solve the problems of undisclosed pyrazole derivatives, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0203] Embodiment 1, intermediate preparation
[0204] (1) Synthesis of intermediate 1-ethyl-3-methyl-5-pyrazole carboxylic acid ethyl ester
[0205]
[0206] 154.1g (1mol) intermediate A is added in the flask of 1000ml, add 500ml acetonitrile and 138g potassium carbonate, then add 1mol diethyl sulfate, the system is stirred and heated to reflux reaction, plate chromatography traces about 3hr reaction is completed, system filtration, The mother liquor was evaporated to dryness with a rotary evaporator, and the residue was distilled under reduced pressure to obtain 140 g of Intermediate B with a yield of 77.0%.
[0207] (2) Synthesis of intermediate 1-ethyl-3-methyl-4-chloro-5-pyrazole ethyl carboxylate
[0208]
[0209] Add 18.2g (0.1mol) of intermediate B to a 100ml flask, add 50ml of dichloroethane and 138g of potassium carbonate, then add 0.11mol of sulfonyl chloride, the system is stirred and heated to reflux, and the reaction is completed by plate chromatography trac...
Embodiment 2
[0249] Embodiment 2, target compound preparation
[0250] (1) Target compound 226
[0251]
[0252] Add 0.31g (0.001mol) of intermediate TA-1 and 0.12g (0.0011mol) of intermediate F-1, 0.15g of sodium carbonate and catalytic amount of sodium iodide into 25ml of acetonitrile, then heat to reflux for 7hrs, and follow the plate chromatography After the reaction was completed, the reaction system was cooled to room temperature, the solid was removed by filtration, acetonitrile was distilled off, and the residue was purified by column chromatography to obtain 0.32 g of the product with a yield of 84%.
[0253] (2) Target compound 251
[0254]
[0255] Add 31g (0.1mol) of intermediate TA-1 and 13g (0.11mol) of intermediate F-2, 15 sodium carbonate and a catalytic amount of sodium iodide into 250ml of acetonitrile, then heat and reflux for 10 hours, and follow the completion of the reaction by plate chromatography , the reaction system was cooled to room temperature, the soli...
Embodiment 3、30
[0285] Embodiment 3, 30% suspending agent
[0286]
[0287]
[0288] Compound 251 and other components were thoroughly mixed to obtain a 30% suspension concentrate. Dilution with water to give a 30% suspension concentrate can give any desired concentration of dilution.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com