Synthesis method of micafungin side chain intermediate
A kind of technology of micafungin and synthesis method, which is applied in the field of medicine and achieves the effect of simple steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0022] The following will clearly and completely describe the technical solutions in the embodiments of the present invention with reference to the accompanying drawings in the embodiments of the present invention. Obviously, the described embodiments are only some, not all, embodiments of the present invention. Based on the embodiments of the present invention, all other embodiments obtained by persons of ordinary skill in the art without creative efforts fall within the protection scope of the present invention.
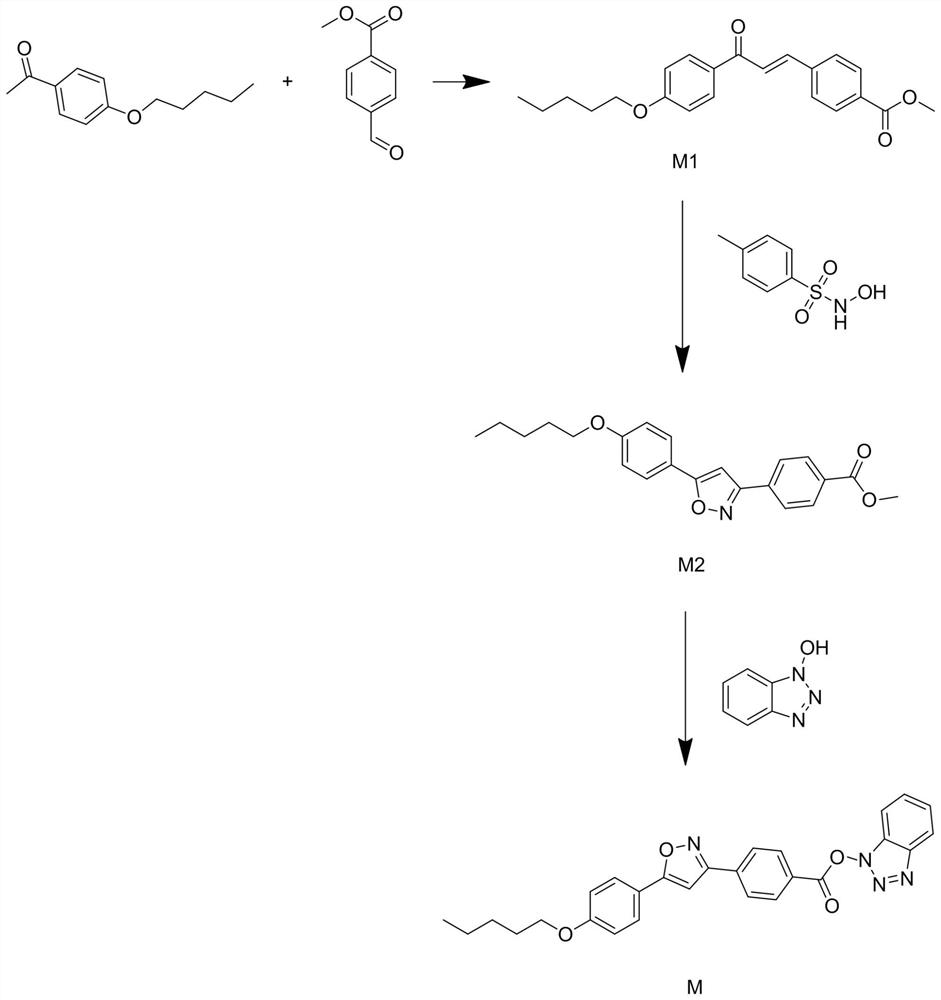
[0023] see figure 1 Shown, the present invention is a kind of synthetic method of micafungin side chain intermediate, specifically comprises the following steps:
[0024] The first step, weigh 50mmol of 4-pentyloxyacetophenone, 51mmol of methyl p-formylbenzoate, 11g of cesium carbonate and 200ml of 55% ethanol aqueous solution into the reaction flask, and heat up to 50°C while stirring , insulated and stirred for 5h, after the reaction was completed, after natural...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com