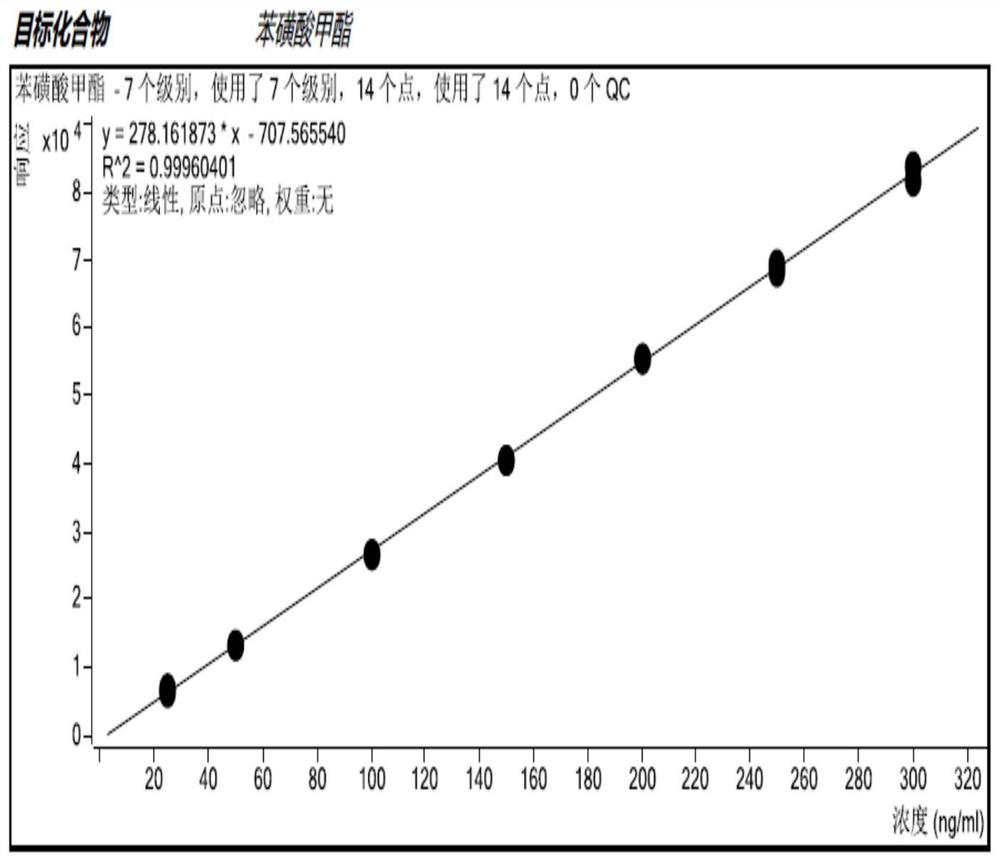
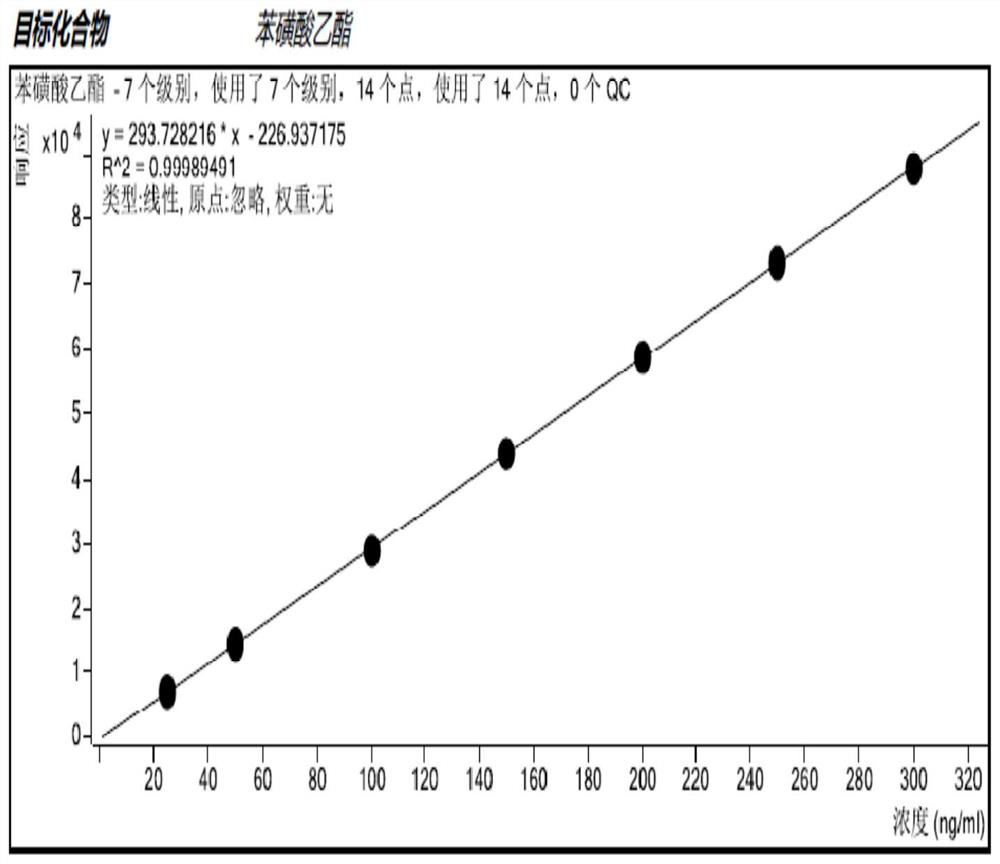
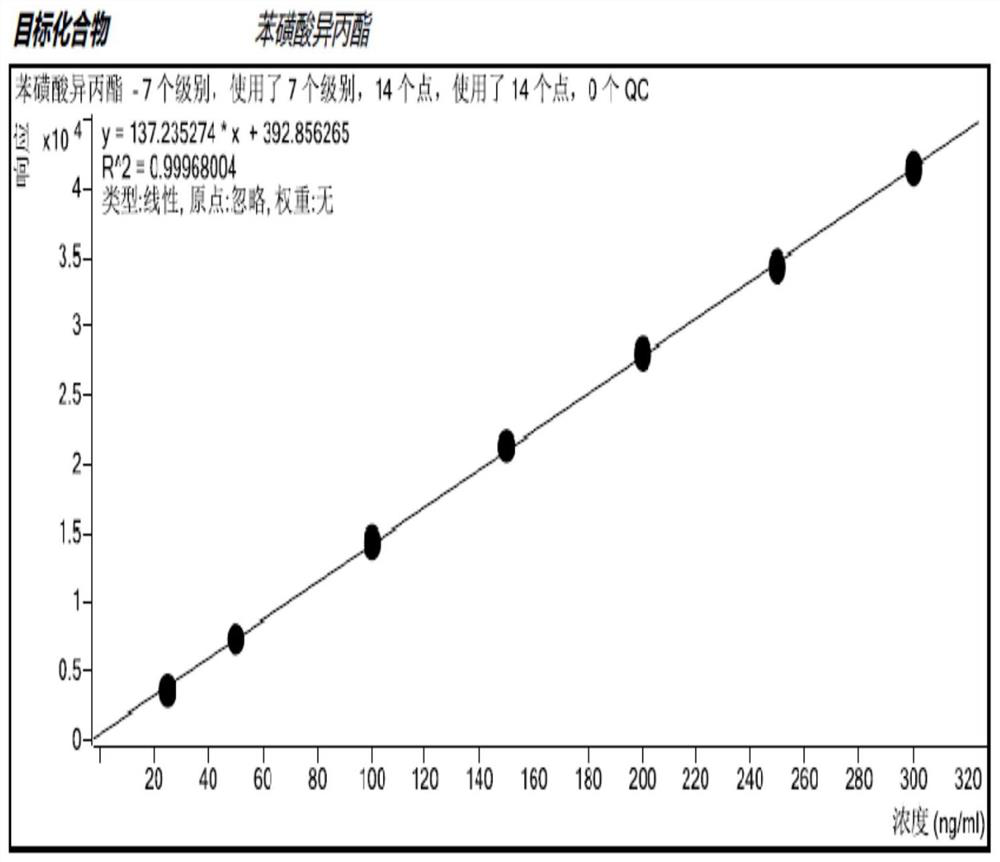
Method for detecting methanesulfonate genotoxic impurities in gemcitabine hydrochloride by GC-MS/MS method
A technology of gemcitabine hydrochloride and mesylate, applied in the field of drug analysis, to achieve the effects of strong specificity, wide linear range and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] 1 Instruments and reagents
[0056] Agilent 7890B-7000C Gas Chromatograph-Tandem Mass Spectrometer
[0057] Dichloroethane source: TCI (Shanghai) Chemical Industry Development Co., Ltd.; batch number: P1529190;
[0058] Ethyl acetate source: LiChrosolv; Lot number: 10942649812; Purity: MS grade
[0059] Anhydrous sodium sulfate: Xilong Science Co., Ltd.; batch number: 1801051; content ≥ 99.0%; purity: analytically pure
[0060] Reference substance source
[0061] Methyl methanesulfonate source: SIGMA-ALORICH; batch number: MKCG1346; content: >99%
[0062] Source of ethyl methanesulfonate: SIGMA-ALORICH; Batch number: BCBW8635;
[0063] Isopropyl mesylate source: Thermo Fisher Scientific (China) Co., Ltd.; batch number: A031422; content: >98.0%
[0064] Propyl mesylate source: TCI (Shanghai) Chemical Industry Development Co., Ltd.; batch number: G6G6L-OM; content: >99%
[0065] Butyl mesylate source: Beijing Bailingwei Technology Co., Ltd.; batch number: LS20S35; c...
Embodiment 2
[0127] According to the preparation method of the test solution provided in Example 1, the quality of the gemcitabine hydrochloride crude drug was changed to 1.1 g, and the rest remained unchanged, and its methodological data could reach the effect of Example 1.
Embodiment 3
[0129] According to the preparation method of the test solution provided in Example 1, the quality of the gemcitabine hydrochloride bulk drug was changed to 1.3 g, and the rest remained unchanged, and its methodological data could reach the effect of Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Column length | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Film thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com