A kind of betahistine synthesis method
A technology of betahistine and synthetic method, which is applied in the field of betahistine synthesis, can solve the problems that the product yield cannot reach this level, strict production conditions, poor control, etc., and achieve high product yield and low reaction temperature Low, good safety performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
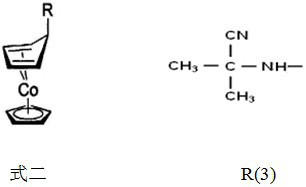
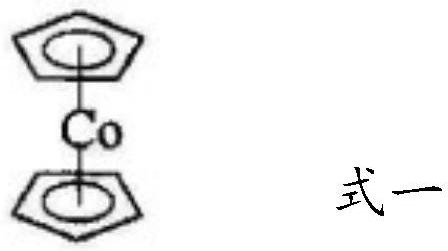
[0048] The method for synthesizing betahistine provided in this embodiment includes the following steps: put 100kg of 3-methylaminopropionitrile, 100kg of xylene, and 0.3kg of cyclopentadienyl cobalt as a catalyst in a 1000L high-pressure reaction kettle, and at a negative pressure of 0.09MPa Next, replace it with high-purity nitrogen three times, pass purified and dry acetylene gas to 0.1MPa, heat it to 70°C, and then continue to feed acetylene gas, keep the temperature at 85°C, and control the pressure at about 0.6MPa. When the acetylene is produced, the pressure in the reactor rises sharply. At this time, the reaction ends, and the injection of acetylene gas is stopped. The pressure drops to 0.08MPa, and the temperature drops to 40°C. Except for the solvent, the crude product of betahistine with a content of 82% and 2 % of the by-product benzene and about 15% of unreacted raw materials and other impurities, the yield is greater than 80%.
Embodiment 2
[0050] The method for synthesizing betahistine provided by this embodiment includes the following steps: put 100kg of 3-methylaminopropionitrile, 600kg of benzene, and 5kg of cyclopentadienyl cobaltocene as a catalyst in a 1000L autoclave, and under a negative pressure of 0.09MPa , replaced three times with high-purity nitrogen, introduced purified and dried acetylene gas to 0.1MPa, heated to 65°C, and then continued to feed acetylene gas, controlled the temperature at 95°C, and controlled the pressure at about 0.8MPa. During acetylene, the pressure in the reaction kettle rose sharply. At this time, the reaction was over, and the injection of acetylene gas was stopped. The pressure dropped to 0.08MPa, and the temperature dropped to 40°C. Except for the solvent, the content of betahistine was 88% crude and 2% betahistine was obtained. The by-product benzene and about 10% unreacted raw materials and other impurities have a yield greater than 85%.
Embodiment 3
[0052] The betahistine synthesis method that the present embodiment provides, comprises the steps: drop into 100kg of 3-methylaminopropionitrile, toluene 250kg, catalyst a 2kg (catalyst a is 1:0.55 bisocene by mol ratio) in the autoclave of 1000L Cobalt and azobisisobutyronitrile are reacted in 5 times the molar amount of tetrahydrofuran), at a negative pressure below 0.09MPa, replace it with high-purity nitrogen three times, pass purified and dry acetylene gas to 0.1MPa, and heat to 65 ℃ , and then continue to feed acetylene gas, control the temperature at 90°C, and control the pressure at about 0.9MPa. When feeding 65kg of acetylene, the pressure in the reactor rises sharply. At this time, the reaction ends, stop injecting acetylene gas, and the pressure drops to 0.08MPa , the temperature was lowered to 40°C, and the content of crude betahistine was 96%, 2% of by-product benzene and 2% of unreacted raw materials and other impurities were obtained except for the solvent, and t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com