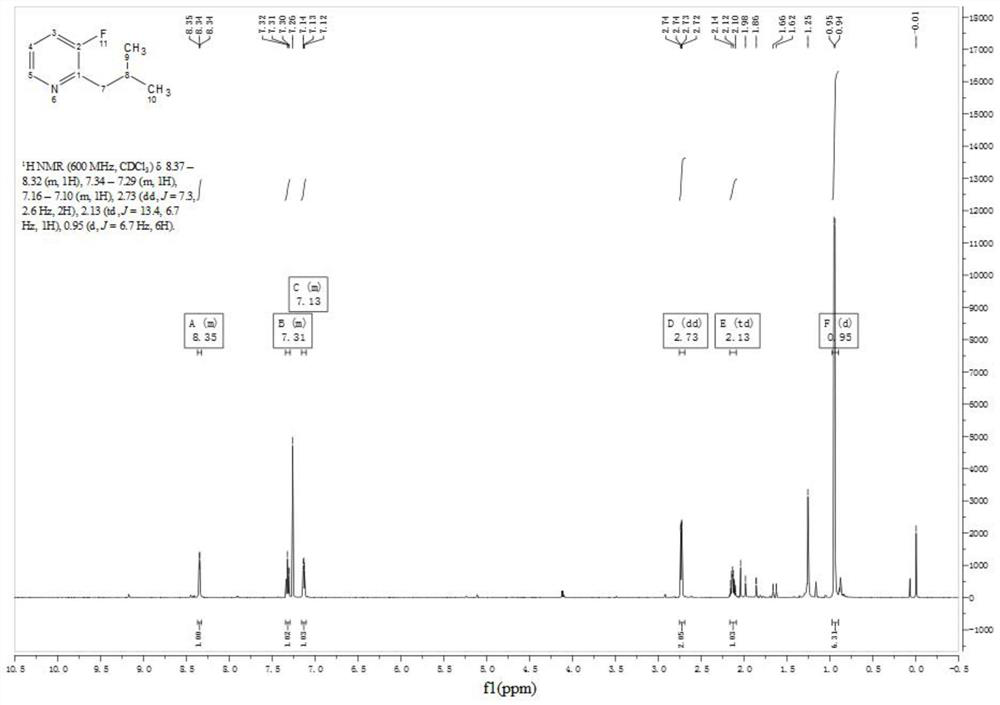
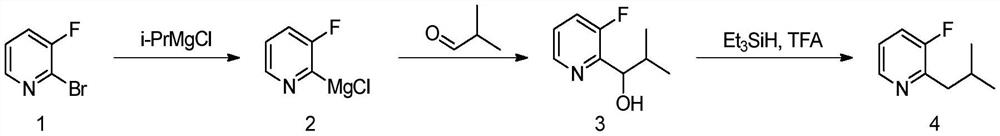
Preparation method of 3-fluoro-2-isobutylpyridine
A technology of isobutylpyridine and isobutyraldehyde, which is applied in the field of preparation of 3-fluoro-2-isobutylpyridine, can solve the problems of high cost of starting materials, high reaction conditions, influence on purification and the like, and achieves affordable production cost. The effect of simple control and post-processing and short synthesis route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] 1) At room temperature and under the protection of argon, add isopropylmagnesium chloride lithium chloride tetrahydrofuran solution (1.3mol / L, 87.4ml, 113.6mmol, 2.0eq), the mixed solution was stirred until complete reaction, general reaction 2h, the color of reaction solution from light to dark; Get small sample and quench with acetone, through small plate TLC (development agent polarity: Petroleum ether / ethyl acetate=3:1) showed that the raw material disappeared, and a new point with large polarity was produced, and the reaction solution containing (3-fluoropyridin-2-yl)magnesium chloride was obtained, because the Grignard reagent was unstable, the The reaction solution was immediately used in the next step to synthesize compound 3.
[0033] 2) Under the protection of 0°C and inert gas, the reaction solution (56.8mmol) containing (3-fluoropyridin-2-yl)magnesium chloride was added dropwise to vigorously stirred isobutyraldehyde (8.2g, 113.6mmol, 2.0eq ) in anhydrous ...
Embodiment 2
[0036] The difference from Example 1 is that when compound 2 is synthesized from compound 1, 2-bromo-3-fluoropyridine (10g, 56.8mmol) anhydrous tetrahydrofuran solution (100mL) is added with isopropylmagnesium chloride lithium chloride tetrahydrofuran solution (1.3 mol / L, 43.7ml, 56.8mmol), the equivalent ratio of reactants was 1:1; the reaction conditions in the preparation process of synthetic compound 3 and compound 4 were unchanged; the yield of the final product 3-fluoro-2-isobutylpyridine was 73.35 %.
Embodiment 3
[0038] The difference from Example 1 is that when compound 3 is synthesized from compound 2, the reaction solution (56.8 mmol) containing (3-fluoropyridin-2-yl) magnesium chloride is added dropwise to vigorously stirred isobutyraldehyde (4.1 g, 56.8 mmol ) in anhydrous tetrahydrofuran solution (200mL), the equivalent ratio of reactants was 1:1; other reaction conditions were unchanged during the preparation of compound 4; the yield of the final product 3-fluoro-2-isobutylpyridine was 60.59%.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap