Group of markers for predicting severe acute viral respiratory infectious diseases as well as application and kit thereof
A viral and respiratory technology, applied in the field of biomedicine, can solve problems such as inability to fully and accurately predict the severity and prognosis of patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0032] In the present invention, the preparation method of the kit preferably includes the following steps:
[0033] (1) Coupling the capture antibodies coated with IL-8, IL-10, GITR, CD28, IL-18 respectively with the encoded microspheres to obtain IL-8, IL-10, GITR, CD28, IL- 18 capture antibody encoded microspheres;
[0034] (2) Link biotin to IL-8, IL-10, GITR, CD28, IL-18 detection antibodies respectively to obtain biotin-labeled IL-8, IL-10, GITR, CD28, IL-18 detection antibodies ;
[0035] There is no sequence relationship between the steps (1) and (2).
[0036] In the present invention, IL-8, IL-10, GITR, CD28, and IL-18 capture antibodies are coupled to encoded microspheres to obtain IL-8, IL-10, GITR, CD28, and IL-18 capture antibodies coated respectively. Encoded microspheres. In the present invention, the coupling method preferably includes the following steps:
[0037] a. Take the carboxyl microspheres and shake the microsphere suspension with a vortex oscilla...
Embodiment 1
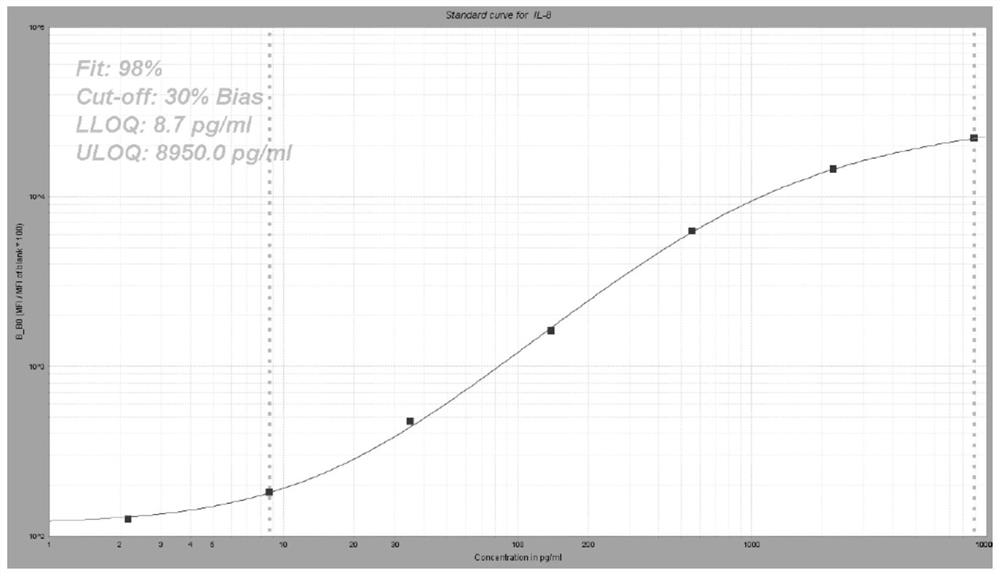
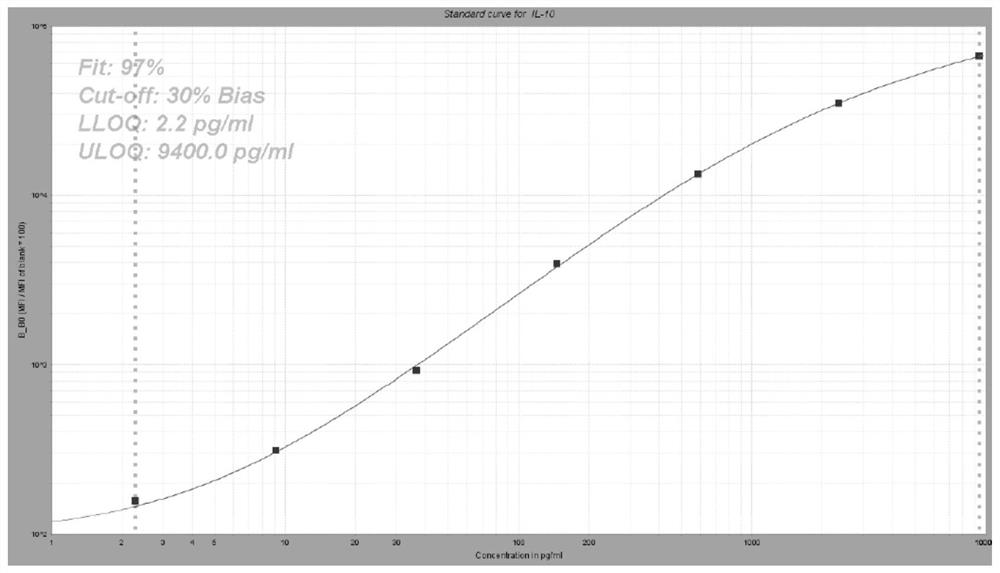
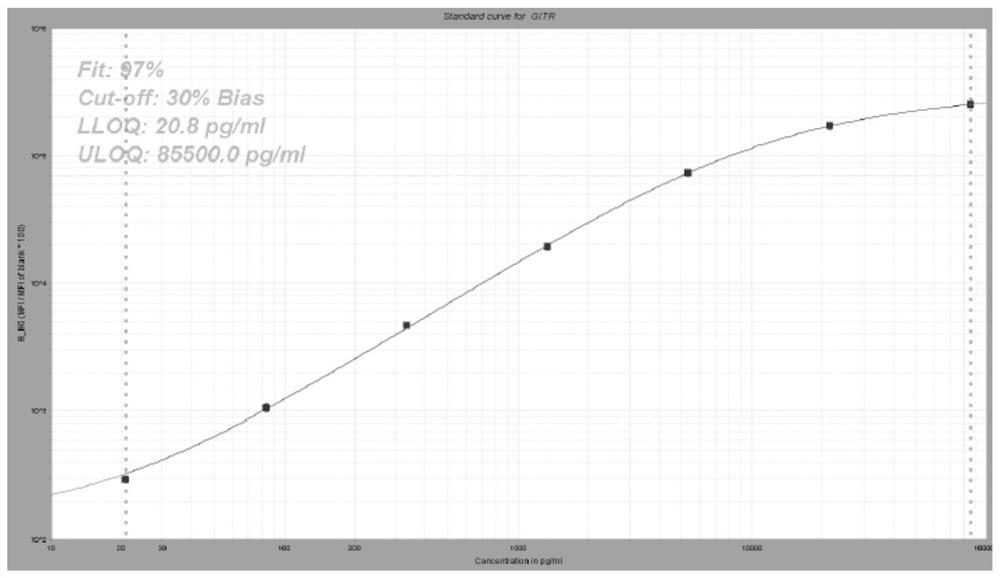
[0061] Preparation of liquid-phase chip kits for detection of IL-8, IL-10, GITR, CD28, IL-18 biomarkers.
[0062] 1. Kit Composition
[0063] (1) 5-plex coated microspheres: containing encoded microspheres coated with IL-8, IL-10, GITR, CD28, and IL-18 capture antibodies respectively;
[0064] (2) 5-plex biotin-labeled detection antibodies: IL-8, IL-10, GITR, CD28, IL-18 detection antibodies labeled with biotin respectively;
[0065] (3) streptavidin phycoerythrin.
[0066]Among them, the clone numbers of capture antibodies IL-8, IL-10, GITR, CD28, and IL-18 are 3IL8-H10, JES3-9D7, 110416, 10F3, and H44, respectively; IL-8, IL-10, GITR, CD28, The clone numbers of IL-18 detection antibodies are I8-S2, BT-10, DT5D3, 37407, and W18160A, respectively.
[0067] 2. Kit Preparation
[0068] Include the following steps:
[0069] (1) The corresponding capture antibody is coated with the corresponding microsphere
[0070] a. Take carboxyl microspheres and vibrate the microsphere s...
Embodiment 2
[0088] Application of IL-8, IL-10, GITR, CD28, IL-18 liquid chip kits in the prediction of acute viral respiratory infectious diseases.
[0089] 1. Purpose of the experiment
[0090] It was proved that the baseline concentrations of IL-8, IL-10, GITR, CD28, and IL-18 at admission were high in severe and critically ill patients.
[0091] 2. Subjects
[0092] 1) On the premise of informed consent and meeting the inclusion conditions, select the enrolled cases and record personal basic information. The cohort of COVID-19 patients came from 83 COVID patients in Beijing Ditan Hospital Affiliated to Capital Medical University, including 6 asymptomatic patients, 53 mild patients, 14 severe patients, and 10 critical patients.
[0093] 2) Plasma was drawn from the patients before treatment when they were admitted to the hospital, and the samples were stored in a -80°C refrigerator.
[0094] 3. Reagent Preparation
[0095] The kit prepared in Example 1 was used.
[0096] (1) Beads:...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com