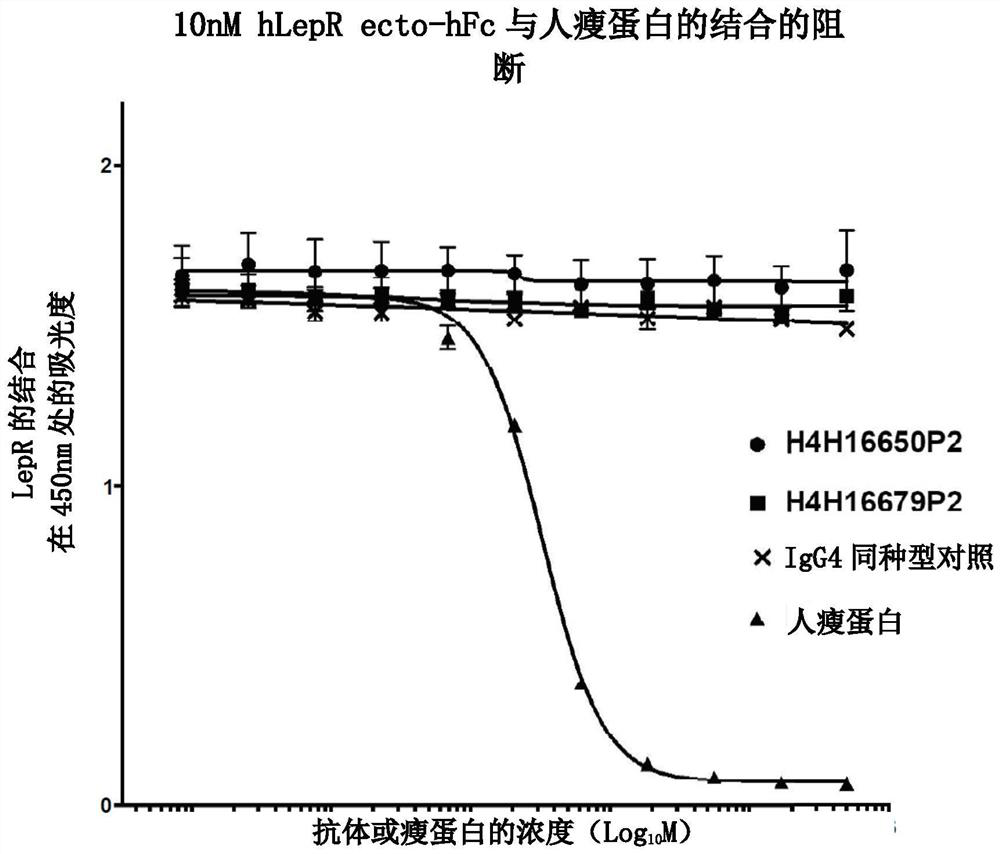
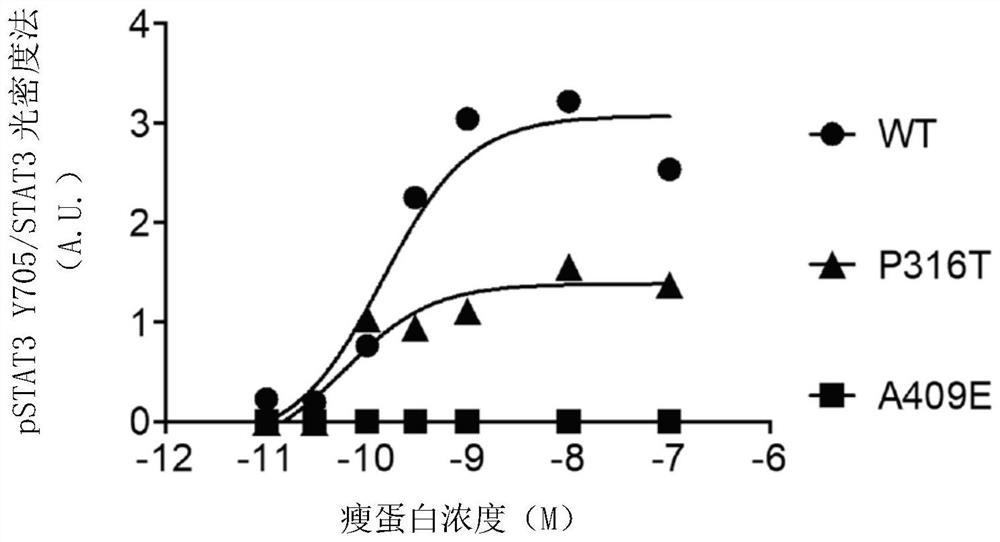
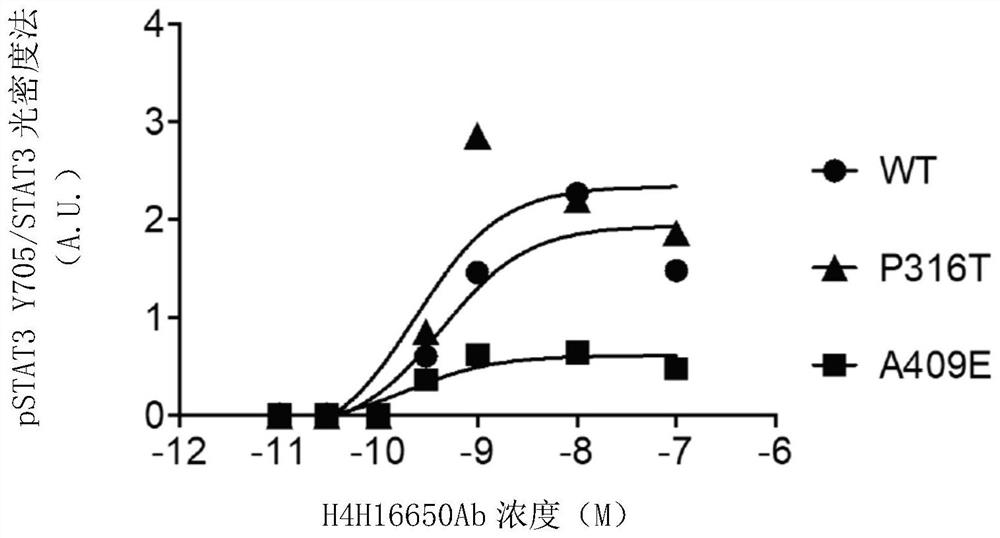
Leptin receptor agonist antibody for use in treating metabolic dysfunction or hypoleptinemia
A technology for metabolic function and protein blood, which is applied in the field of restoring insulin sensitivity and can solve problems such as limited efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0142] Production of Human Antibodies
[0143] Anti-LEPR antibodies of the invention may be fully human antibodies. Methods for producing monoclonal antibodies, including fully human monoclonal antibodies, are known in the art. Any such known methods may be used in the context of the present invention to prepare human antibodies that specifically bind to human LEPR.
[0144] Use e.g. VELOCIMMUNE TM technology, or any other similar known method for producing fully human monoclonal antibodies, initially isolates high affinity chimeric antibodies to LEPR having human variable regions and mouse constant regions. Antibodies are characterized and selected for desired characteristics, including affinity, ligand blocking activity, selectivity, epitope, etc., as described in the Experimental section below. Fully human anti-LEPR antibodies are generated by replacing the mouse constant regions, if desired, with the desired human constant regions (eg, wild-type or modified IgGl or IgG4...
Embodiment 1
[0205] Example 1. Generation of Antigen Binding Proteins that Specifically Bind Leptin Receptor (LEPR)
[0206] by immunization with an immunogen comprising the extracellular domain of LEPR Mice (ie, mice engineered to contain DNA encoding human immunoglobulin heavy chain and kappa light chain variable regions) were used to obtain anti-LEPR antibodies. Antibody immune responses were monitored by LEPR-specific immunoassays. Fully human anti-LEPR antibodies were isolated and purified using previously described techniques.
[0207] Certain biological properties of exemplary anti-LEPR antibodies produced according to the methods of this example are described in detail in the Examples presented below.
Embodiment 2
[0208] Example 2. Heavy and light chain variable region amino acid and nucleic acid sequences
[0209] Table 1 shows the amino acid sequence identifiers of the heavy and light chain variable regions and CDRs of selected anti-LEPR antibodies of the invention. Table 2 shows the corresponding nucleic acid sequence identifiers.
[0210] Table 1: Amino Acid Sequence Identifiers
[0211]
[0212]
[0213] Table 2: Nucleic acid sequence identifiers
[0214]
[0215]Antibodies are generally referred to herein according to the following nomenclature: an Fc prefix (eg, "H4H", "H1M", "H2M", etc.), followed by a numerical identifier (eg, "16650", "16679", etc.), followed by a "P" or "N" suffix. Accordingly, according to this nomenclature, antibodies may be referred to herein, for example, as "H4H16650P2", "H4H16679P2", etc. The Fc prefix (H4H, H1M, and H2M) on antibody names as used herein indicates the specific Fc region isotype of the antibody. For example, an "H4H" antibo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com