Sanguinarine derivatives, chelerythrine derivatives and application thereof
A technology of chelerythrine and derivatives, applied in the directions of application, botanical equipment and methods, biocides, etc., to achieve the effects of excellent insecticidal activity, strong biological activity, and strong insecticidal activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
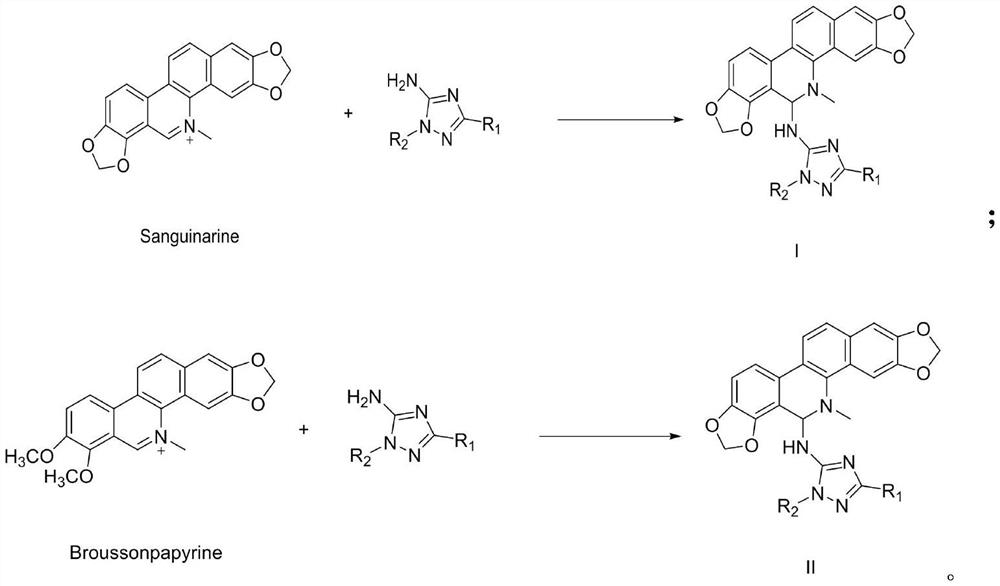
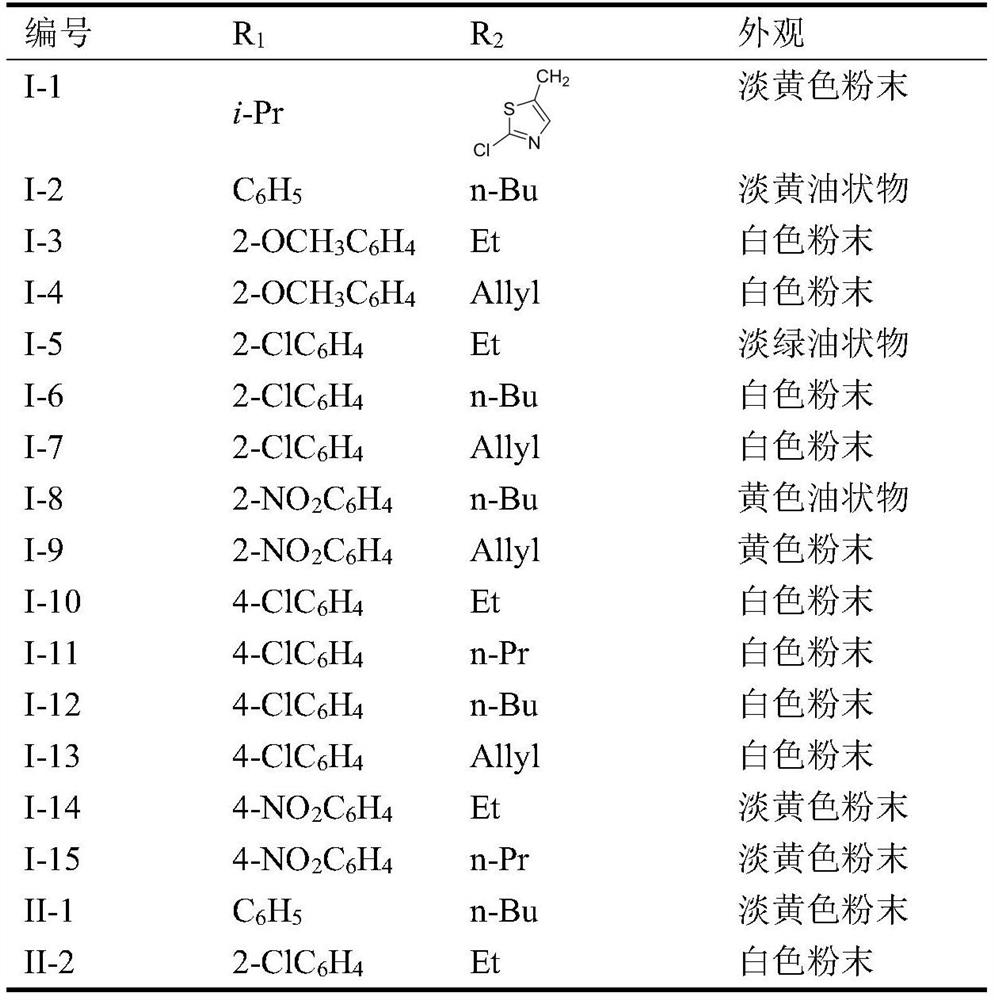
[0030] Embodiment 1: the preparation of compound 1-2
[0031] In a 250mL three-necked flask, add bolus sanguinarine (1g, 0.003mol), acetonitrile 50mL, and triethylamine (3eq.) to dissolve under magnetic stirring, then slowly add 1-n-butyl-3-phenyl-1H- 1,2,4-triazol-5-amine (0.0036mol, 1.2eq, 0.82g), after the addition is complete, stir at room temperature for 5h, after TLC detects that the reaction is complete, stop stirring, after the reaction solution is concentrated under reduced pressure, use V Ethyl acetate: V petroleum ether = 1:4 Flash column chromatography gave 0.70 g of light yellow oil with a yield of 43%.
[0032] Structural analysis of compound I-2: 1 H NMR (CDCl 3 ,300MHz)δ6.77(s,1H, NH),6.07(s,2H,CH 2 ),6.13(s,2H,CH2),5.04(s,1H,CH), 8.07~7.80(m,4H,Ph),7.51~7.38(m,4H,Ph),7.18~6.95(m,2H, Ph), 4.47 (d, J = 7.2Hz, 2H, CH2), 3.02 (s, 3H, CH3), 1.95 (d, J = 7.2Hz, 2H, CH 2 ), 0.89(t,J=7.50Hz,3H,CH 3 ); 13C NMR (CDCl 3 , 75MHz) Δ101.1.1.1.2, 144.5,148.5,147.4,148...
Embodiment 2
[0033] The preparation of embodiment 2 compound I-15
[0034] In a 250mL three-necked flask, add bolus sanguinarine (1g, 0.003mol), acetonitrile 50mL, and triethylamine (3eq.) to dissolve under magnetic stirring, then slowly add 1-n-propyl-3-(4-nitro Phenyl)-1H-1,2,4-triazol-5-amine (0.0036mol, 0.9g, 1.2eq), after the addition was completed, stirred at room temperature for 7h, after TLC detected that the reaction was complete, the stirring was stopped, and the reaction solution reduced After concentration under reduced pressure, flash column chromatography with V ethyl acetate: V petroleum ether = 1:4 gave 0.70 g of light yellow oil with a yield of 40%.
[0035] Structural analysis of compound I-15: 1 H NMR (CDCl 3 ,300MHz)δ6.79(s,1H, NH),6.07(s,2H,CH 2 ),6.12(s,2H,CH 2 ),5.04(s,1H,CH),8.07~7.81(m,4H,Ph),7.50~7.39(m,5H,Ph),7.18~6.97(m,2H,Ph),4.46(d,J= 7.2Hz, 2H, CH 2 ),3.02(s,3H,CH 3 ), 1.95 (d, J=7.2Hz, 2H, CH 2 ),1.26~1.25(m, 2H,CH 2),0.89(t,J=7.50Hz,3H,CH 3 ); 13...
Embodiment 3
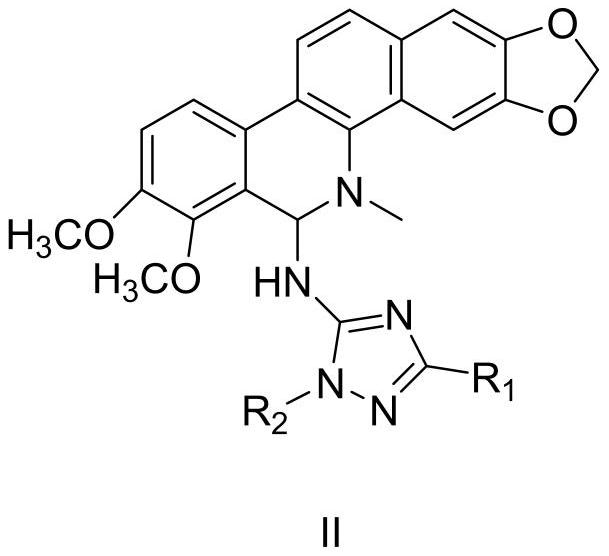
[0036] Embodiment 3: the preparation of compound II-2
[0037] In a 250mL three-necked flask, add chelerythrine (1g, 0.003mol), 50mL of acetonitrile, triethylamine (3eq.) and dissolve under magnetic stirring, then slowly add 1-ethyl-3-(2- Chlorophenyl)-1H-1,2,4-triazol-5-amine (0.0036mol, 0.85g, 1.2eq), after the addition is complete, stir at 60°C for 4h, after TLC detects that the reaction is complete, stop stirring, and react After the solution was concentrated under reduced pressure, it was subjected to column chromatography with V ethyl acetate: V petroleum ether = 1:3 to obtain 0.60 g of white powder with a yield of 36%.
[0038] Structural analysis of compound I-15: 1 H NMR (CDCl 3 ,300MHz)δ6.79(s,1H, NH),6.13(s,2H,CH 2 ),5.06(s,1H,CH),7.81~7.61(m,4H,Ph),7.48~7.38(m,4H,Ph),7.18~6.97(m,2H,Ph),3.68(s,3H, CH 3 ),3.82(s,3H,CH 3 ), 3.97(d,J=7.2Hz,2H,CH 2 ),3.02(s,3H,CH 3 ), 1.54(t, J=7.51Hz, 3H, CH 3 ); 13 C NMR (CDCl 3 ,75MHz)δ101.2,147.4,148.1,158.3,160.1,132.2,1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com