Clinical research data integration platform
A technology that integrates platforms and research data, applied in the field of medical clinical research, can solve the problems of lack of management platform and strong privacy of hospital diagnosis and treatment data, and achieve the effect of ensuring the accuracy of data
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
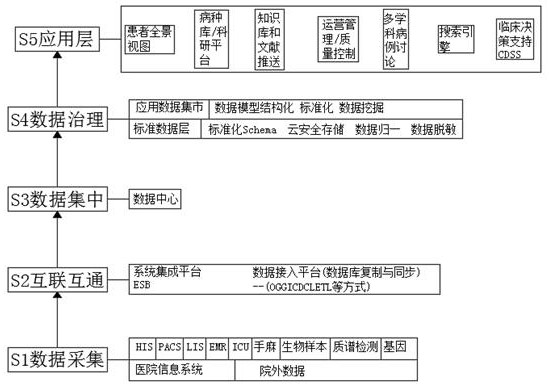
[0054] refer to Figure 1-6 , a clinical research data integration platform, including the following modules:
[0055] A data acquisition module, which includes establishing a data set;
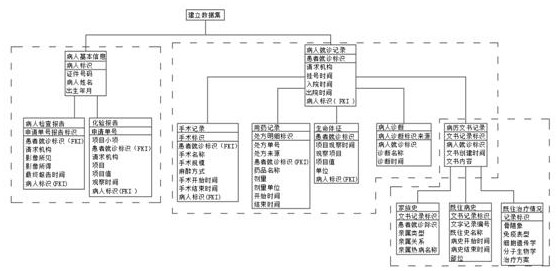
[0056] The established data set includes the following information: basic patient information, patient visit records;
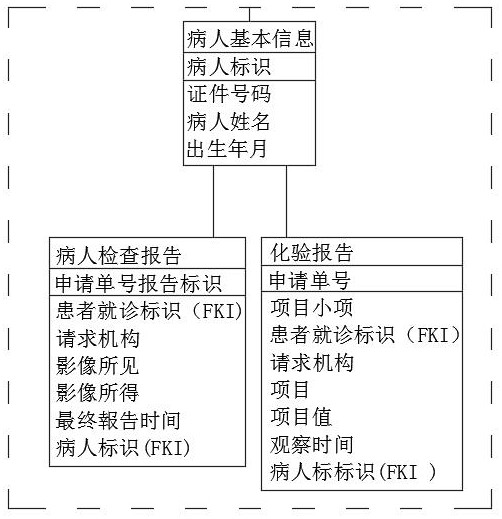
[0057] The patient's basic information includes the certificate number, patient's name, date of birth, and forms the patient's identification;
[0058] The patient's visit record includes the requesting institution, registration time, admission time, discharge time, patient identification (FKI), and forms the patient's visit identification;
[0059] The patient's basic information also includes the patient's examination report and laboratory report;
[0060] The patient examination report includes the patient's visit identification (FKI), requesting agency, imaging findings, imaging results, final report time, patient identification (FKI), and forms the application number...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com