Patents
Literature
89 results about "Patient Visit" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
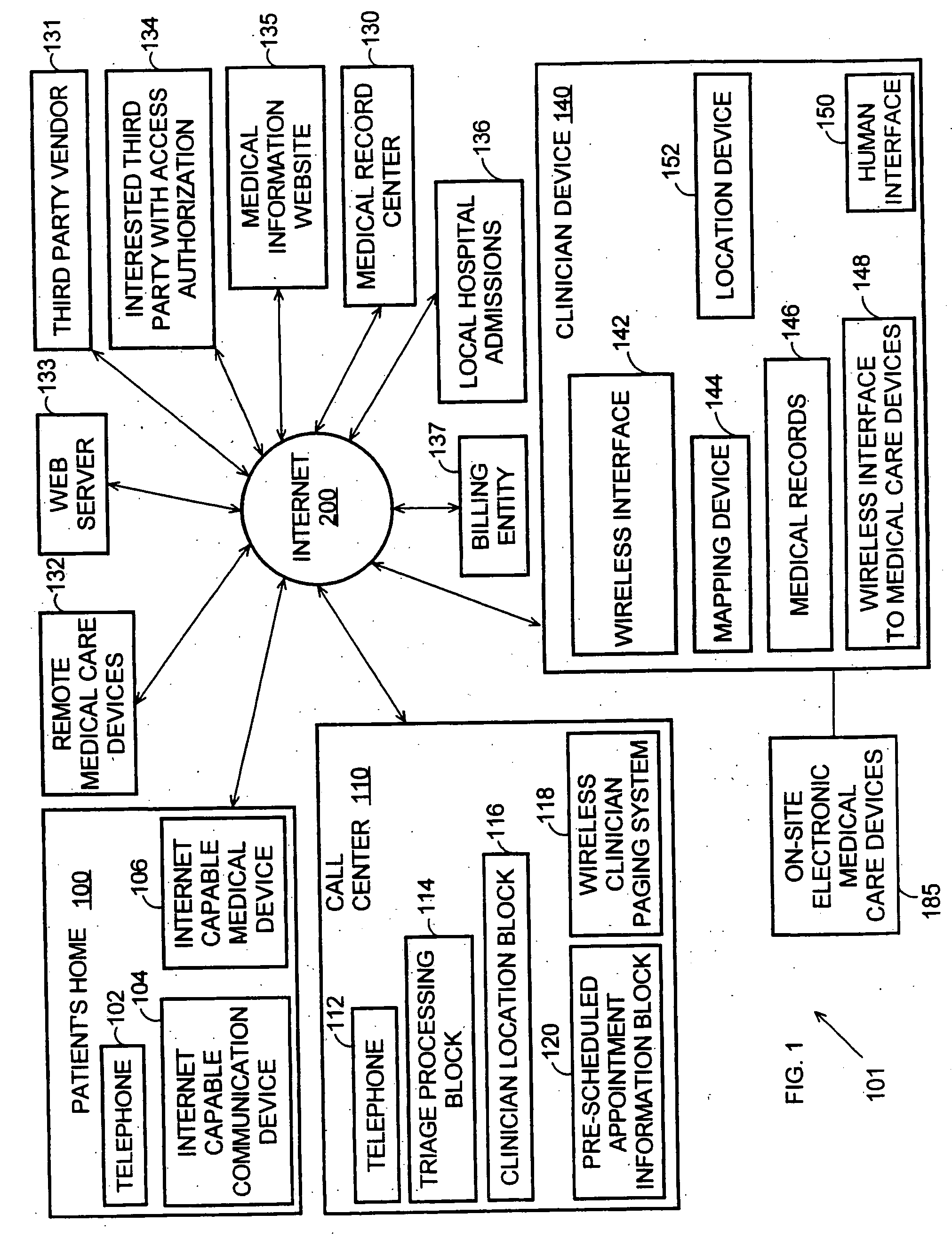
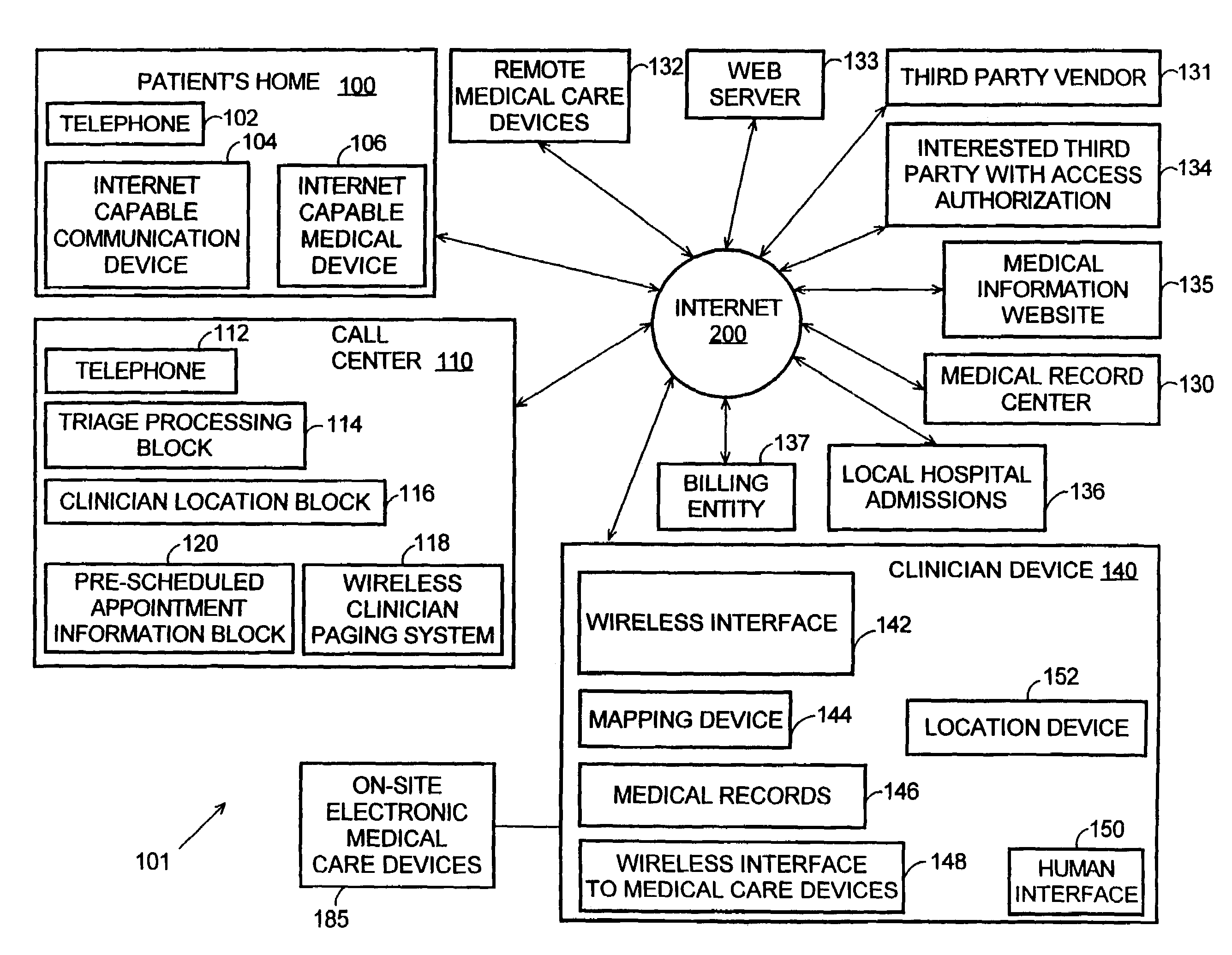
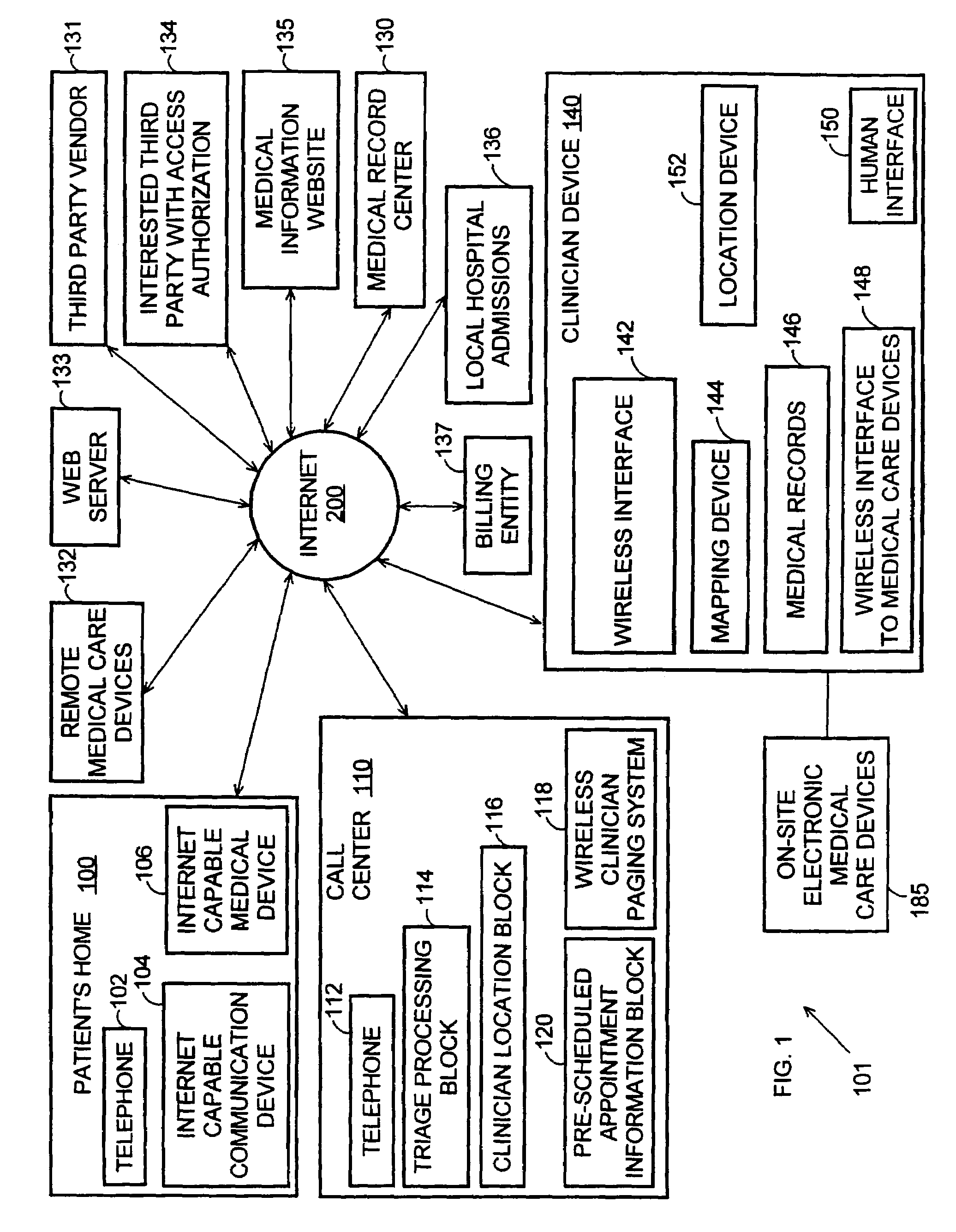
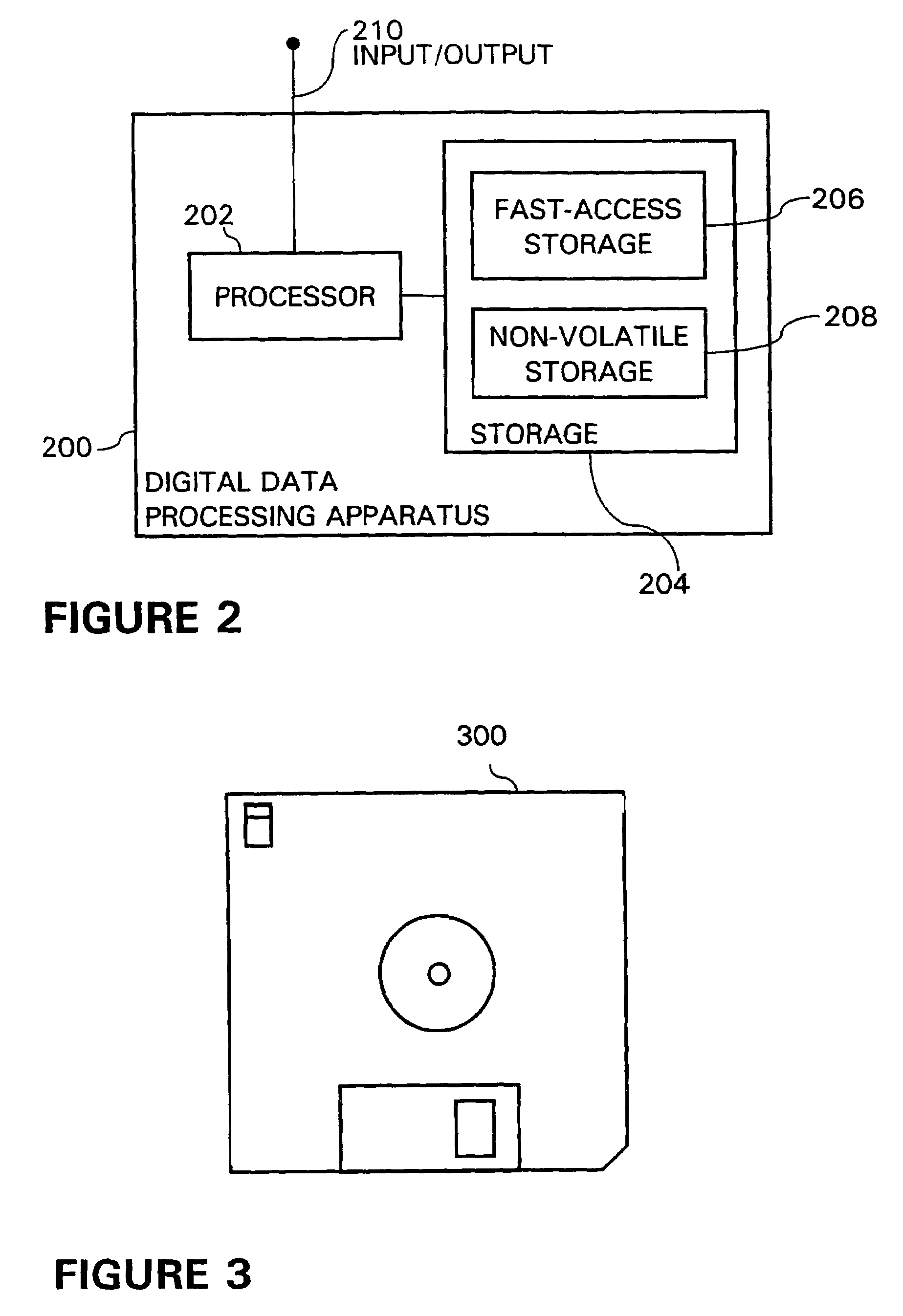
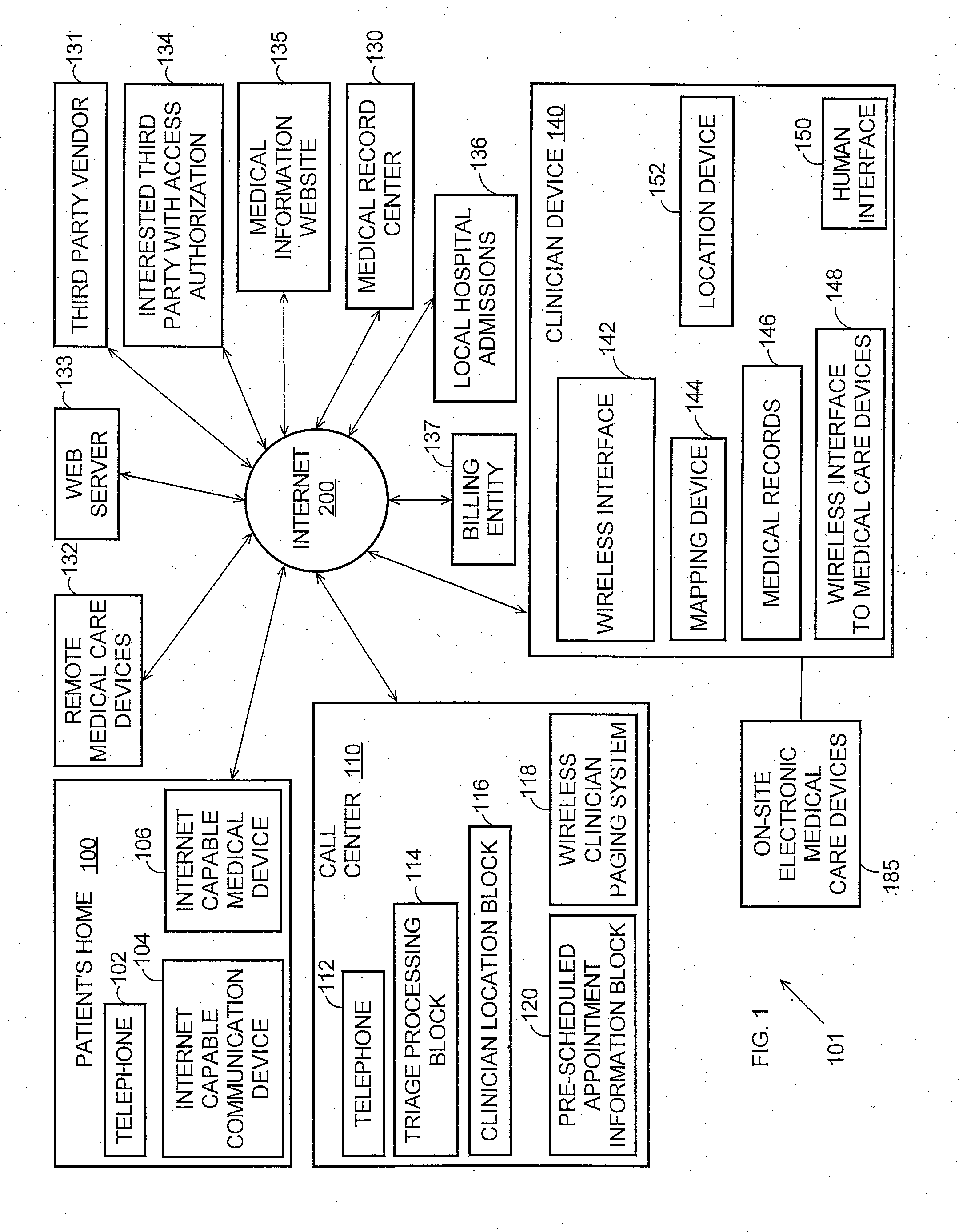
Systems and methods for automatically collecting, formatting, and storing medical device data in a database
InactiveUS20050192844A1Patient personal data managementOffice automationObjective measurementMedical device
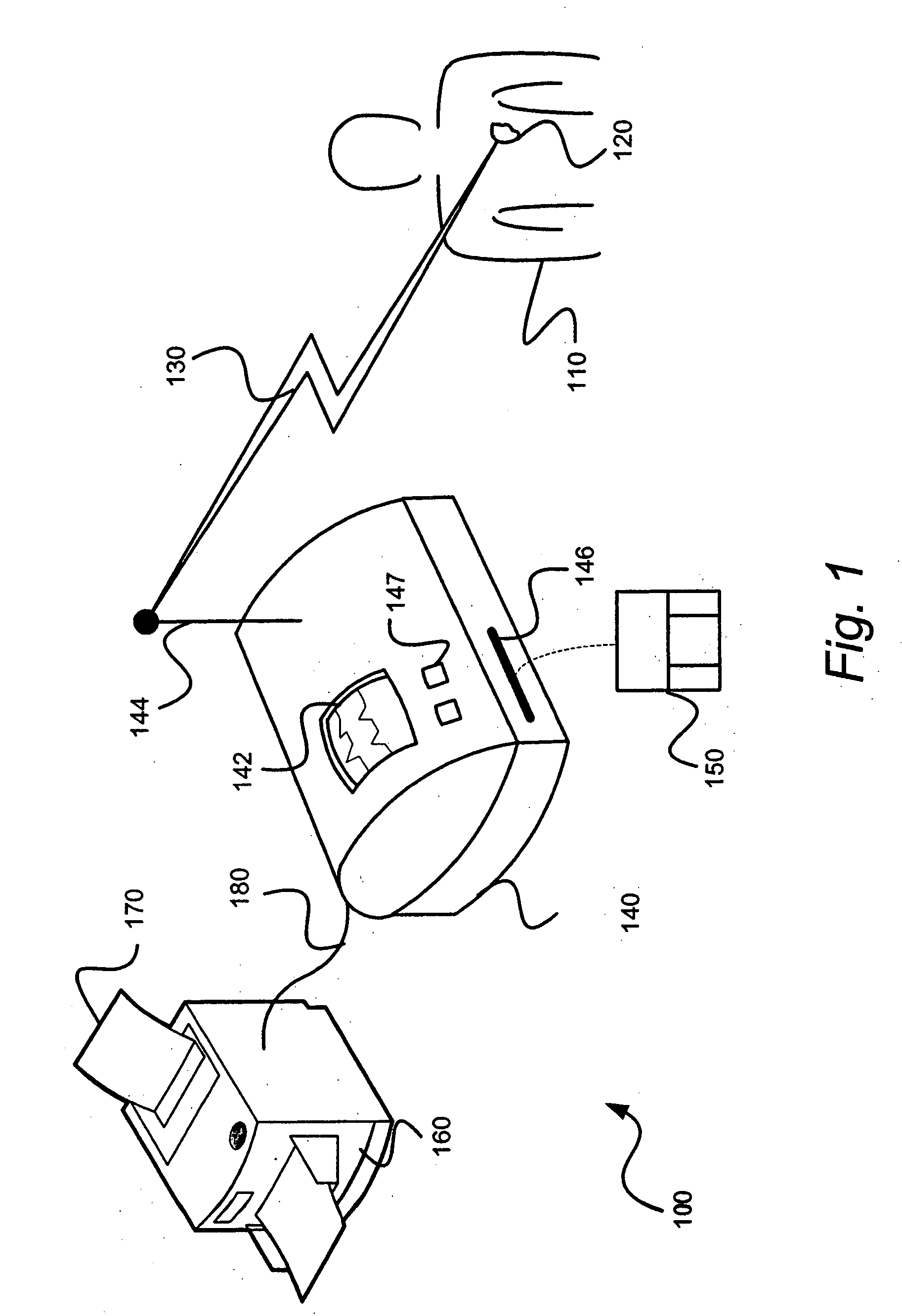
The present invention provides systems and methods for gathering, formatting, storing and / or distributing medical information. In one exemplary embodiment, the present invention provides for centralized maintenance of medical information derived from a patient visit, and for distributed access to various medical information by, for example, researchers, the patient, the patient's physician, a specialist, and / or other recipients. Thus, for example, a patient may visit their physician and during that visit the physician may read information from an implantable medical device associated with the patient, make other objective measurements of the patient, and record various subjective information about the patient. All of this information can be uploaded and maintained on a variably accessible system.
Owner:CARDIAC PACEMAKERS INC
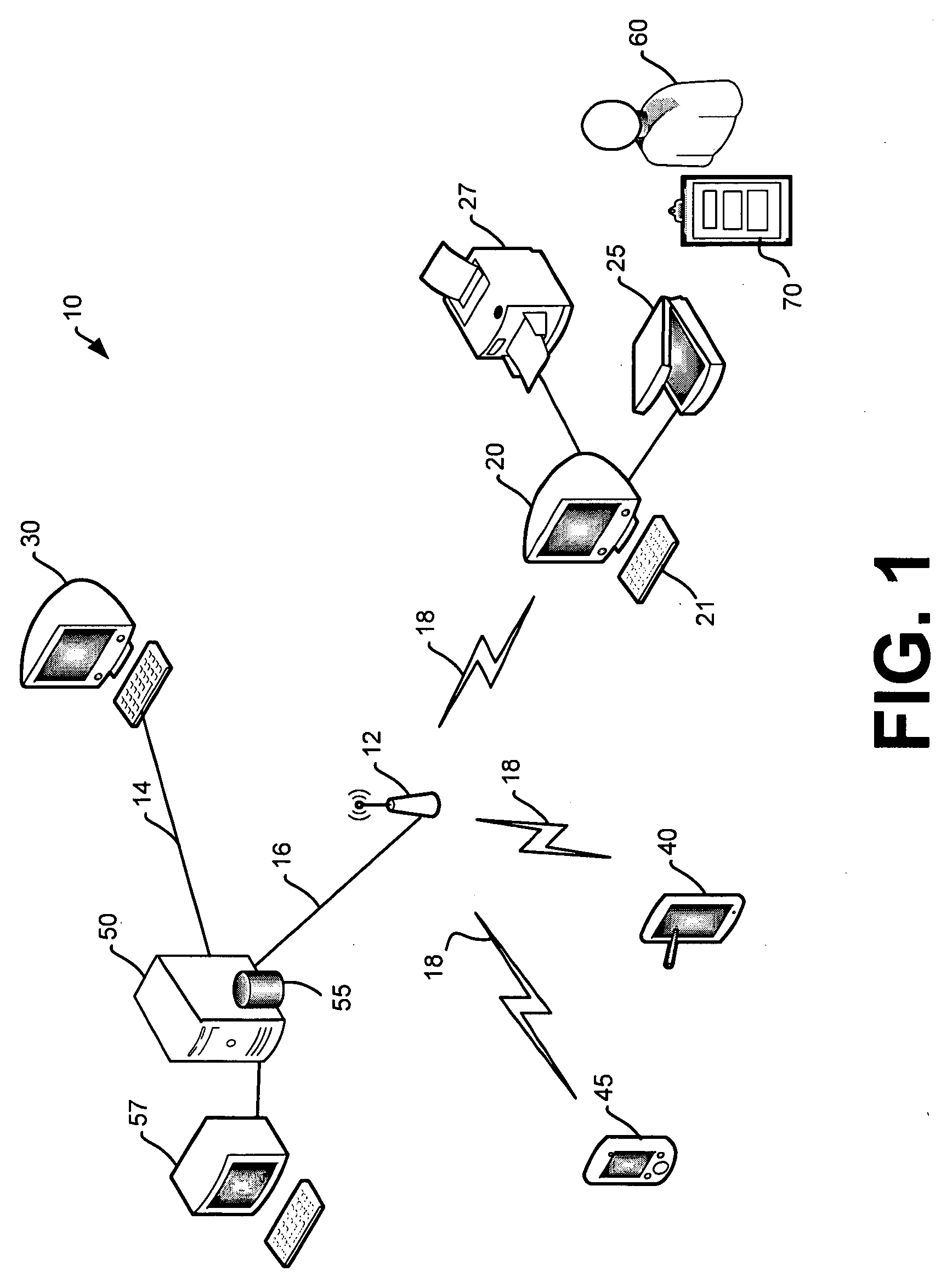
Method for clinician house calls utilizing portable computing and communications equipment
InactiveUS20050060198A1Improve bindingShorten the timeMedical communicationData processing applicationsMedical recordTherapeutic Devices
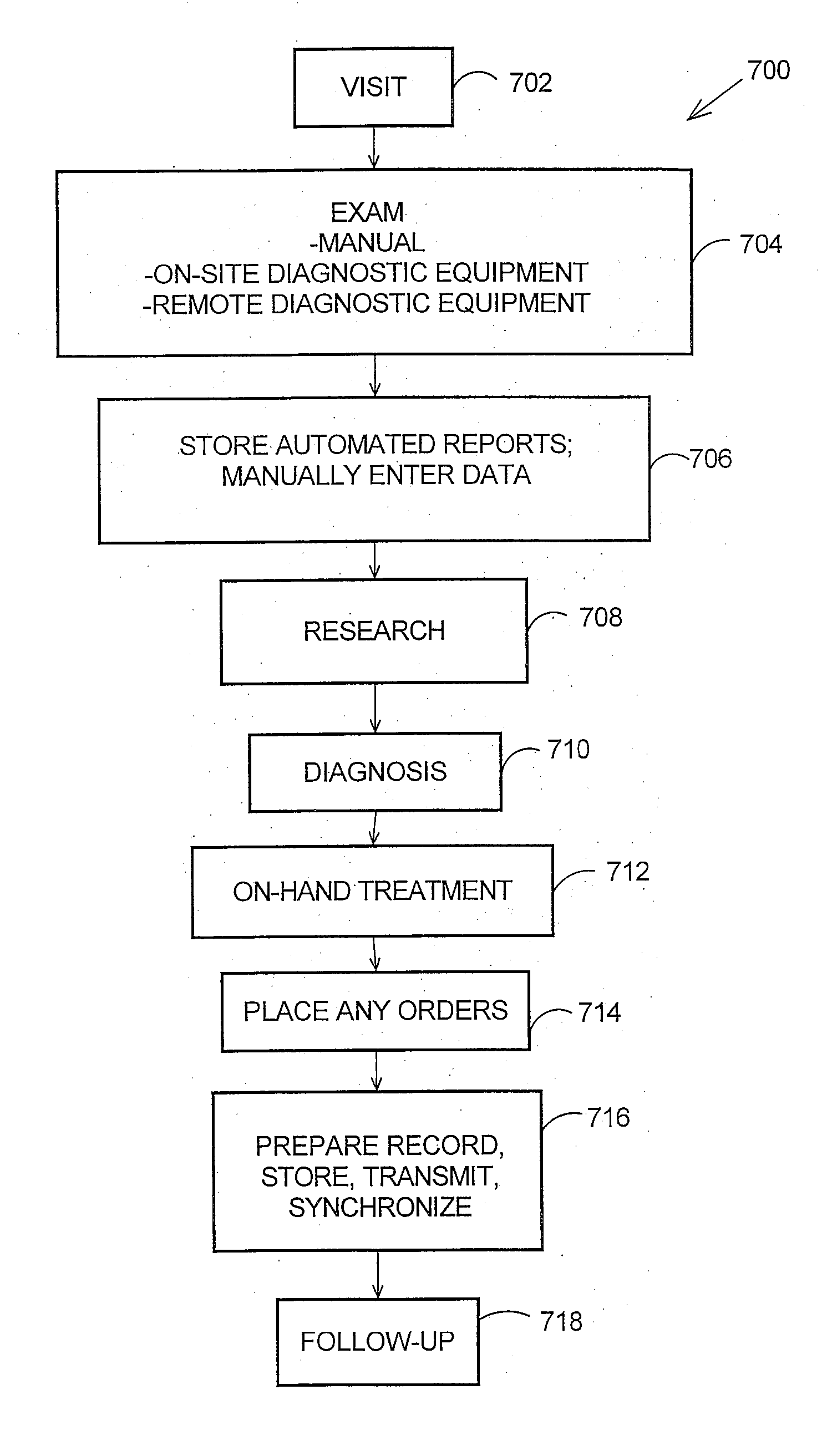
Mobile clinicians conduct in-home patient visits utilizing on-site diagnostic and treatment equipment, where service is enhanced by the use of portable computing and communications equipment. Initially, a mobile care entity provides a network of predesignated mobile clinicians, each having the use of a preprogrammed portable computer. Each computer is coupled to a wireless communications device, and includes local storage of patient data. Under a predetermined schedule, each portable computer updates patient data in the local storage utilizing the wireless communications device to download updates from a central storage facility. Whenever the mobile care entity receives requests for medical service at a patient's premises, the entity selects a mobile clinician and dispatches him / her to the patient's premises. The clinician visits the patient's premises accompanied by an assortment of electronic diagnostic and treatment devices, such as a pulse oximiter, x-ray machine, lab analyzer, EKG equipment, etc. To examine the patient, the doctor utilizes various diagnostic devices to prepare machine-readable reports of related aspects of the patient's condition. The clinician directs the portable computer to perform follow-up tasks including: (1) electronically collecting the prepared reports and graphically presenting them in human-readable form, (2) storing a machine-readable medical record including details of the patient's exam, and (3) utilizing the wireless device to transmit the reports and records to the central storage facility.
Owner:OPTIMA DIRECT LLC
Method for clinician house calls utilizing portable computing and communications equipment
InactiveUS7249036B2Improve bindingShorten the timeMedical communicationData processing applicationsTherapeutic DevicesHouse call
Mobile clinicians conduct in-home patient visits utilizing on-site diagnostic and treatment equipment, where service is enhanced by the use of portable computing and communications equipment. Initially, a mobile care entity provides a network of predesignated mobile clinicians, each having the use of a preprogrammed portable computer. Each computer is coupled to a wireless communications device, and includes local storage of patient data. Whenever the mobile care entity receives requests for medical service at a patient's premises, the entity selects a mobile clinician and dispatches him / her to the patient's premises. The clinician visits the patient's premises accompanied by an assortment of electronic diagnostic and treatment devices. To examine the patient, the doctor utilizes various diagnostic devices to prepare machine-readable reports of related aspects of the patient's condition.
Owner:OPTIMA DIRECT LLC
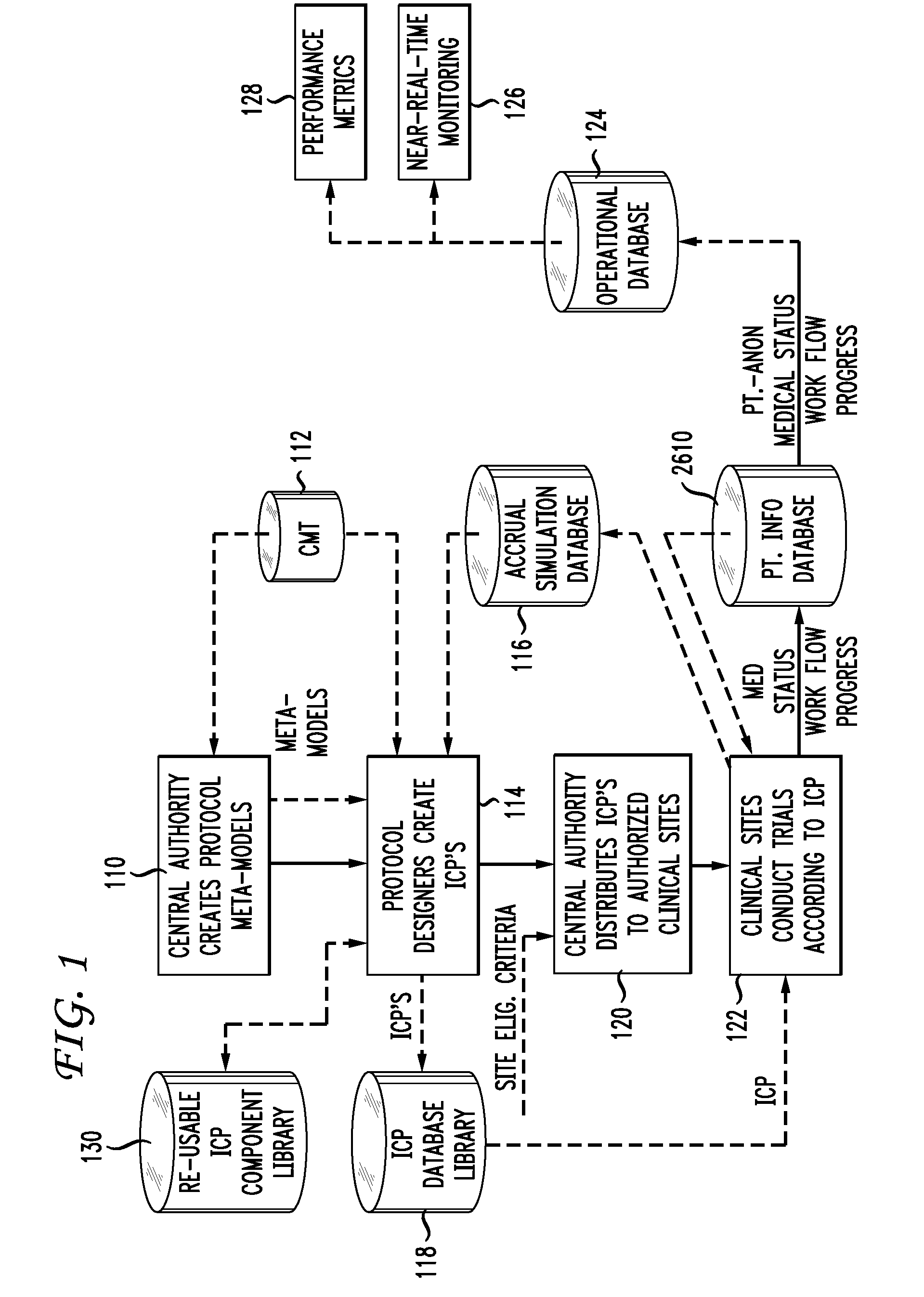
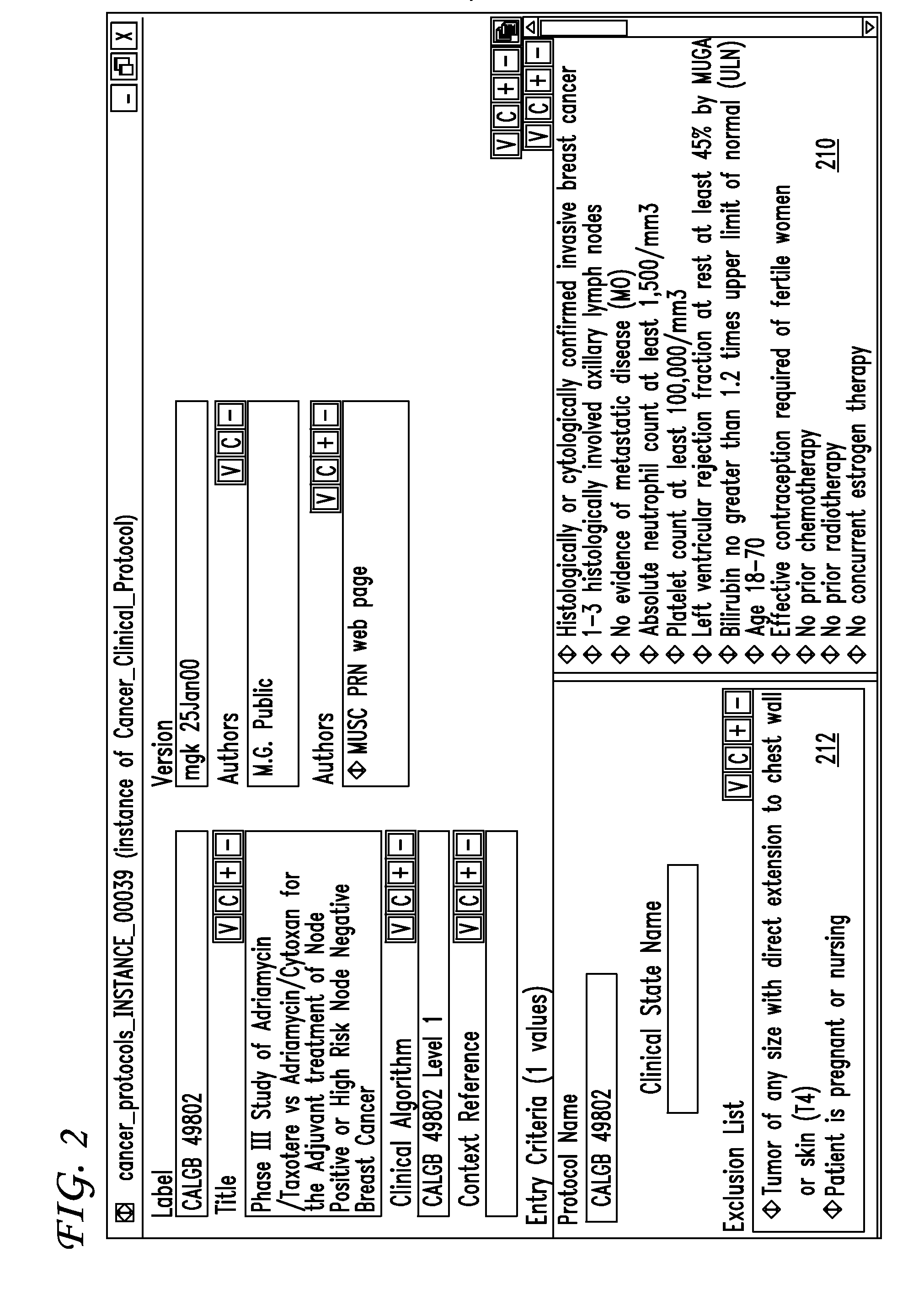
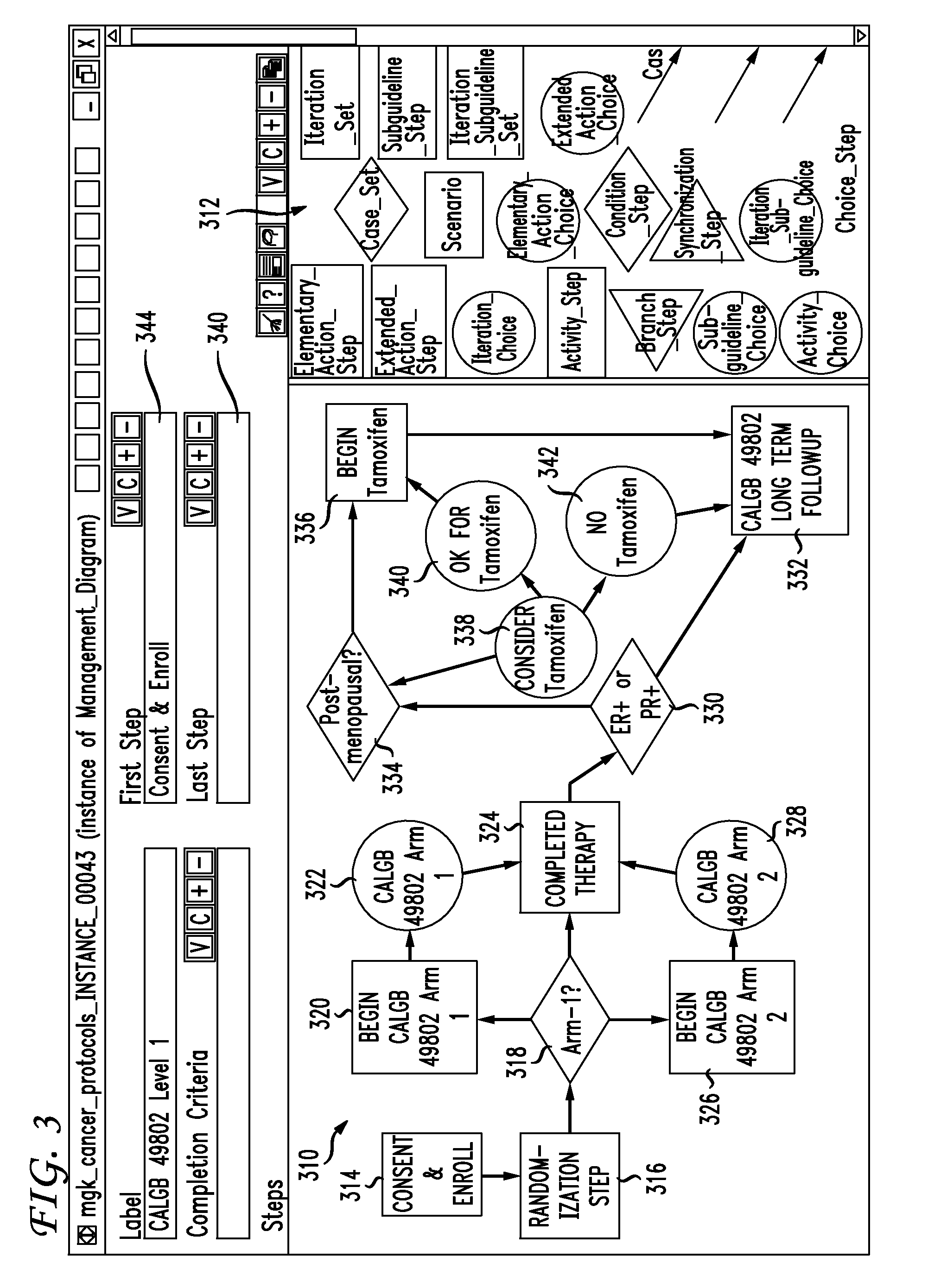
Clinical trials management system and method
InactiveUS20090313048A1Digital data processing detailsDrug and medicationsPatient managementEnd to end system
Clinical trials are defined, managed and evaluated according to an overall end-to-end system. The central authority creates protocol meta-models and makes them available to clinical trial protocol designers. Each meta-model includes a short list of preliminary patient eligibility attributes which are appropriate for a particular disease category. The protocol designer chooses the appropriate meta-model, and encodes the clinical trial protocol, including eligibility and patient workflow, within the selected meta-model. The resulting protocol database is stored together with databases of other protocols in a library of protocol databases. Sponsors and individual clinical sites have controlled access to the protocols. Study sites make reference to the pertinent protocol databases to which they have access in the protocol database library in order to perform patient eligibility screening. Once a patient is enrolled into a study, the protocol database indicates to the clinician what tasks are to be performed at each patient visit. These tasks can include both patient management tasks and data management tasks. The workflow graph advantageously also instructs the proper time for the clinician to obtain a patient's informed consent. The system reports patient progress to study sponsors, who can then monitor the progress of the trial, and to a central authority which can then generate performance metrics. Advantageously, a common controlled medical terminology database is used by all components of the system.
Owner:MEDIDATA SOLUTIONS
Clinical Cost Control Management Module
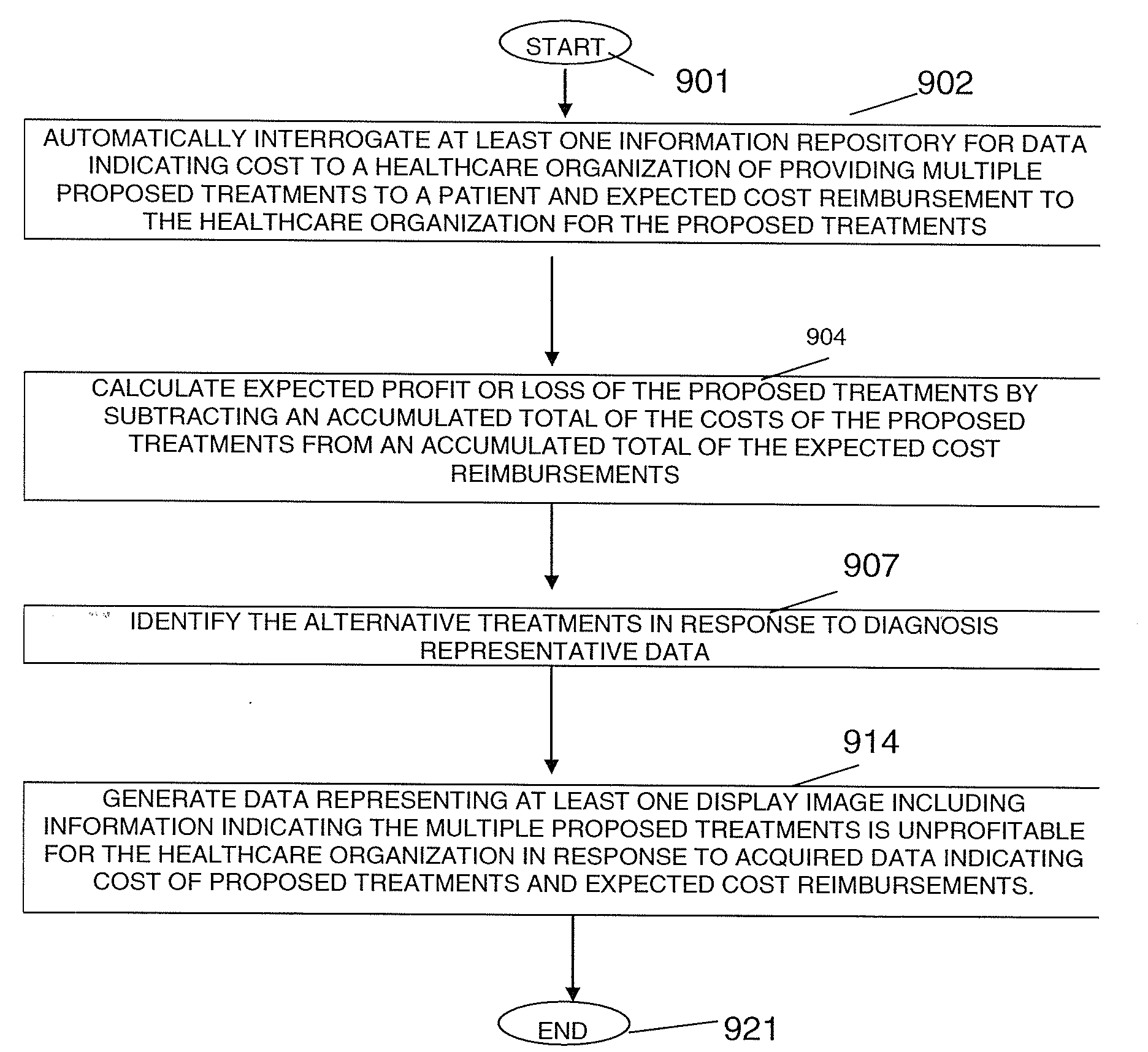
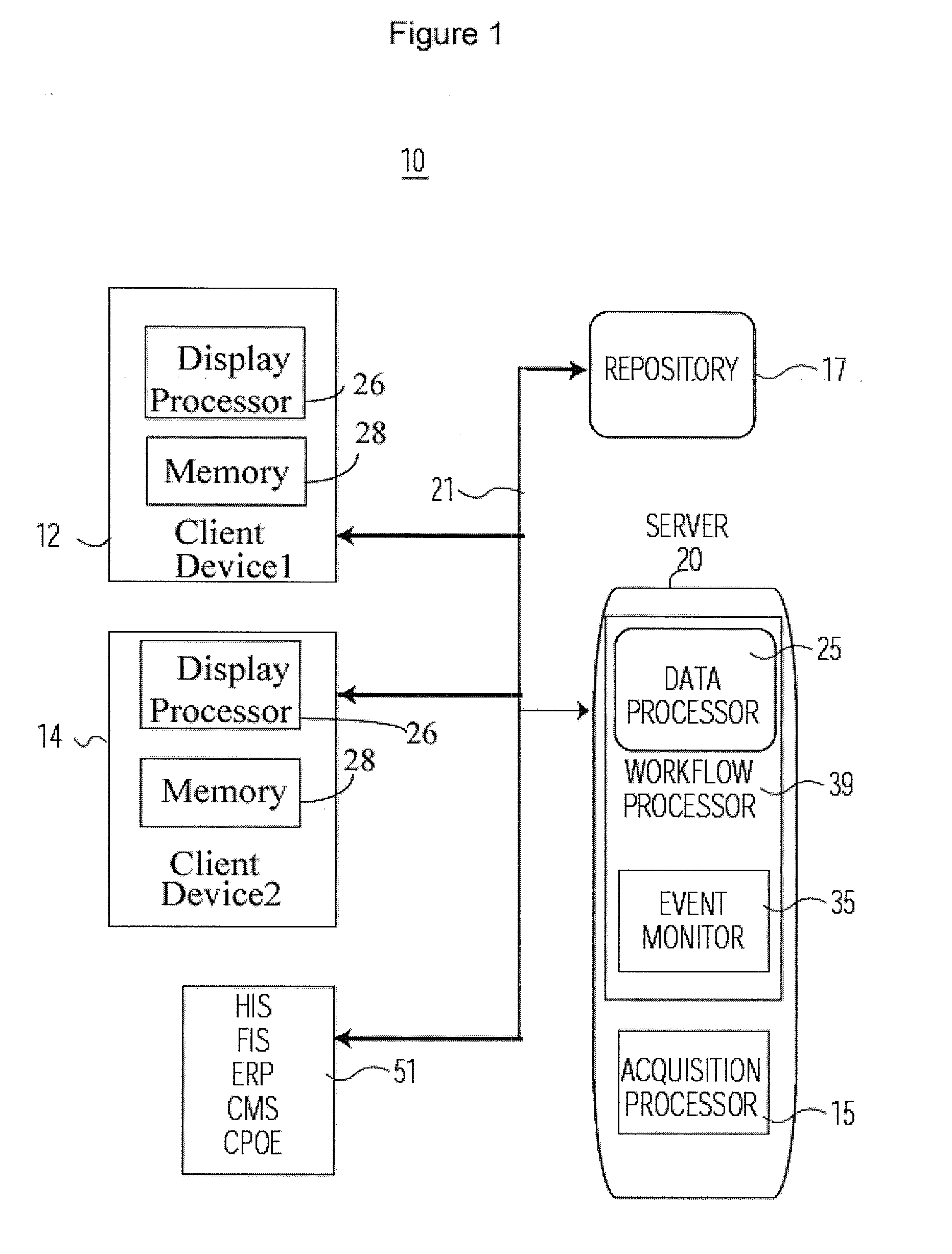
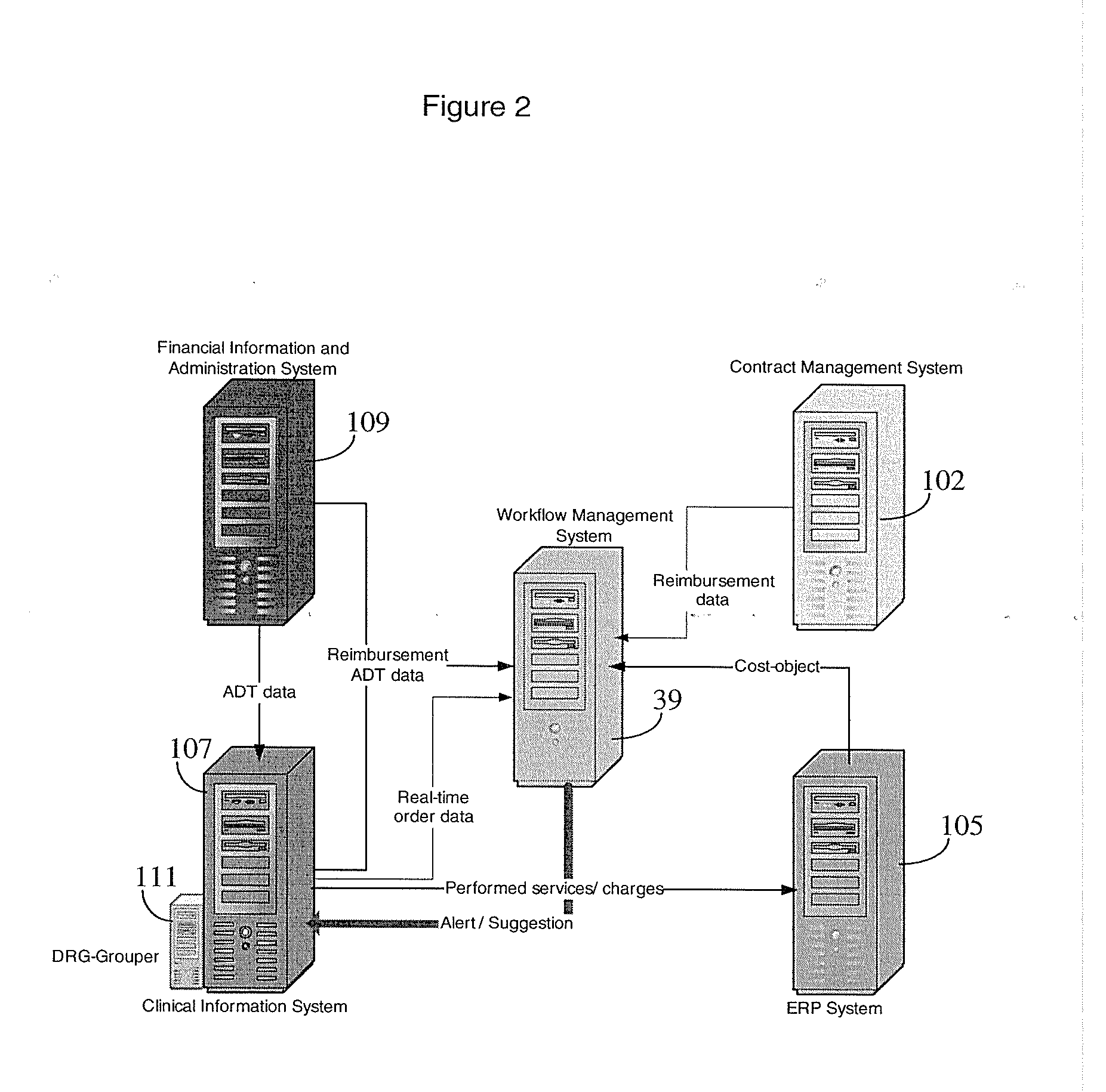
A system enables a healthcare organization to evaluate cost efficient treatment options early in a patient treatment process by automatic monitoring of profit or loss of a treatment, in real-time and enables a clinical user to screen, simulate and control treatment profit or loss during a patient visit, based on both expected reimbursement and the actual costs of performed services. A system enables cost based treatment selection by providing a healthcare worker with patient specific treatment cost information during a treatment episode. An acquisition processor automatically interrogates at least one information repository for data concerning cost to a healthcare organization of providing a proposed treatment to a patient and expected cost reimbursement to the healthcare organization for the proposed treatment. The acquisition processor initiates generation of at least one message including a patient identifier and treatment identification code for communication to the at least one information repository, in response to data indicating occurrence of a treatment related event. A display processor initiates generation of data representing at least one display image including information indicating the proposed treatment is unprofitable for the healthcare organization.
Owner:SIEMENS MEDICAL SOLUTIONS USA INC
Method for clinician house calls utilizing portable computing and communications equipment
InactiveUS20080004907A1Improve bindingShorten the timeDiagnostic recording/measuringRadio/inductive link selection arrangementsMedical recordTherapeutic Devices
Owner:OPTIMA DIRECT LLC
Integrated Record System and Method
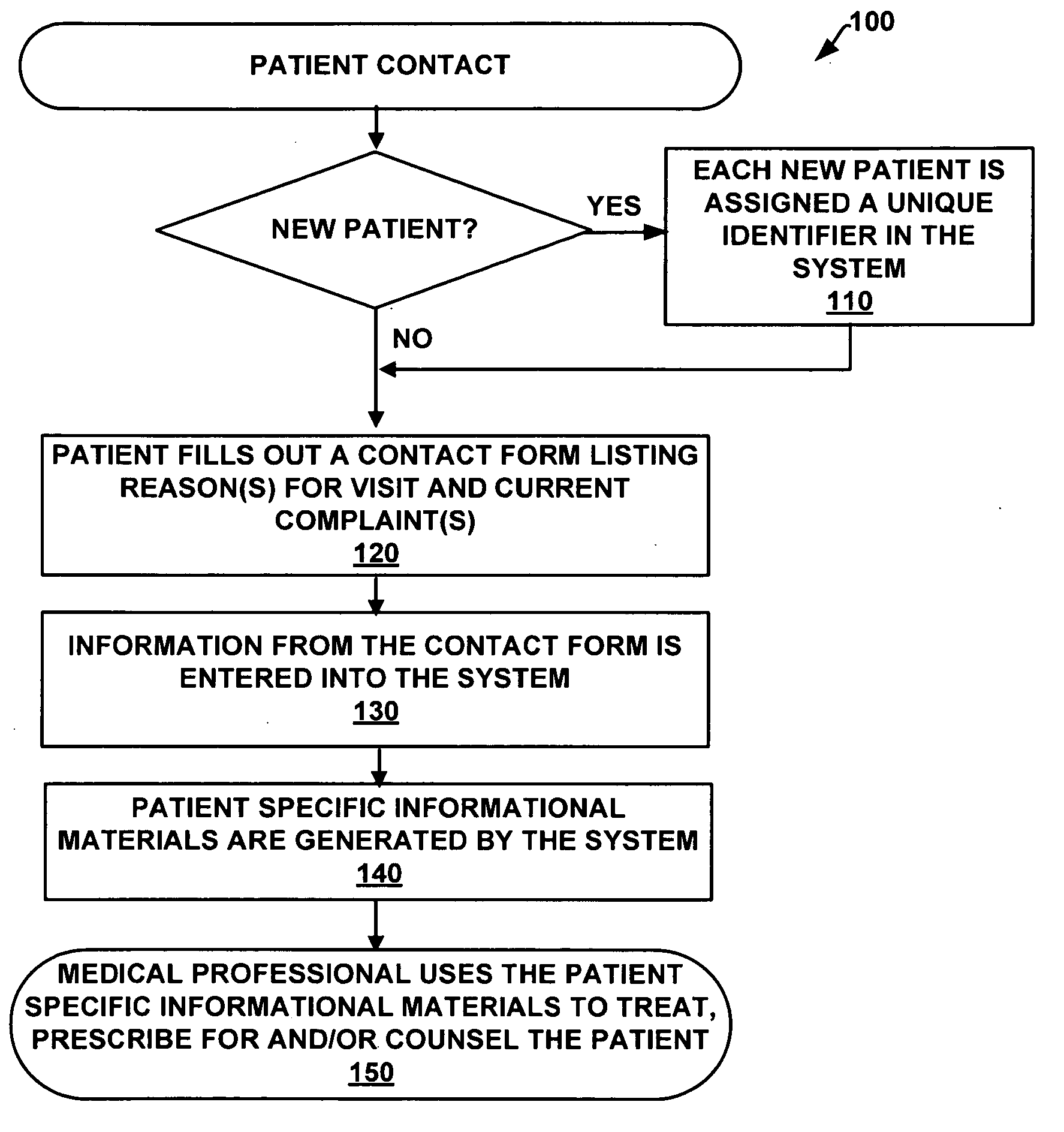
InactiveUS20090138284A1Easy to trackCharacter and pattern recognitionPatient personal data managementMedical recordComputer science
An integrated records system and method is provided that permits rapid encounter recording via a simplified, customized, flexible encounter form uniquely generated on a per-customer basis, and additionally providing unique hand-written and / or dictated impressions. In the case of medical records, the system can be used to generate patient specific encounter forms for use during a patient visit.
Owner:HYBRID MEDICAL RECORD SYST
Cloud platform based medical system case information storage and calling method
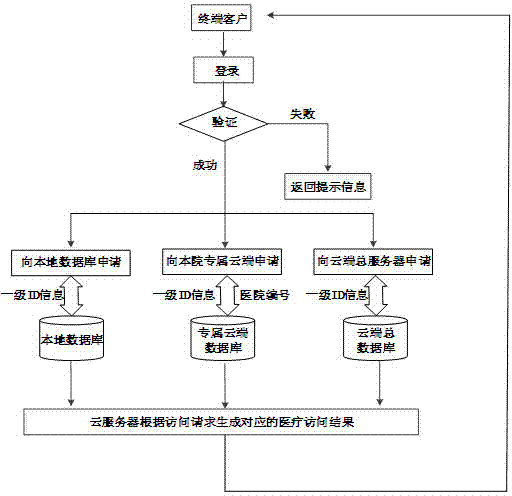
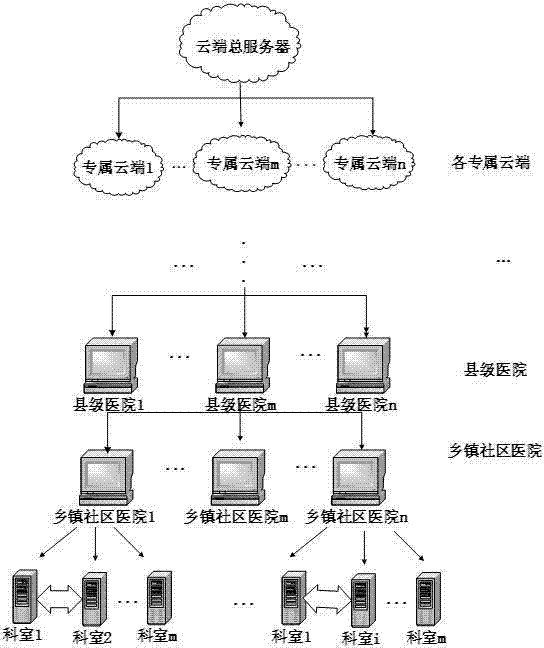
ActiveCN104766024AEnsure safetyEliminate redundancyDigital data protectionSpecial data processing applicationsLower gradeLow graded
The invention discloses a cloud platform based medical system case information storage and calling method and relates to the field of information storage and safety. Hospitals are divided into a plurality of grades, the lower-grade hospitals transmit medical information to the subordinate upper-grade hospitals, the upper-grade hospitals summarize and upload the medical information to a cloud exclusive database to which the upper-grade hospitals belong, and the cloud exclusive database saves stored medical information into a cloud total database. By the aid of the method, the hospitals are graded and upload the total medical information grade by grade to be summarized in the cloud total database finally, patients only fills in personal basic information during visit for the first time if the patients visit the same hospitals for multiple times, and the patients can use patient ID card which the patients already have and identifying the patient identities uniquely, accordingly, time for filling in personal information during registration visit queuing is shortened, screening steps for storage information screening are reduced, information interaction of all hospitals and all departments is achieved, and the problem of 'information island' is solved.
Owner:HENAN QUNZHI INFORMATION TECH
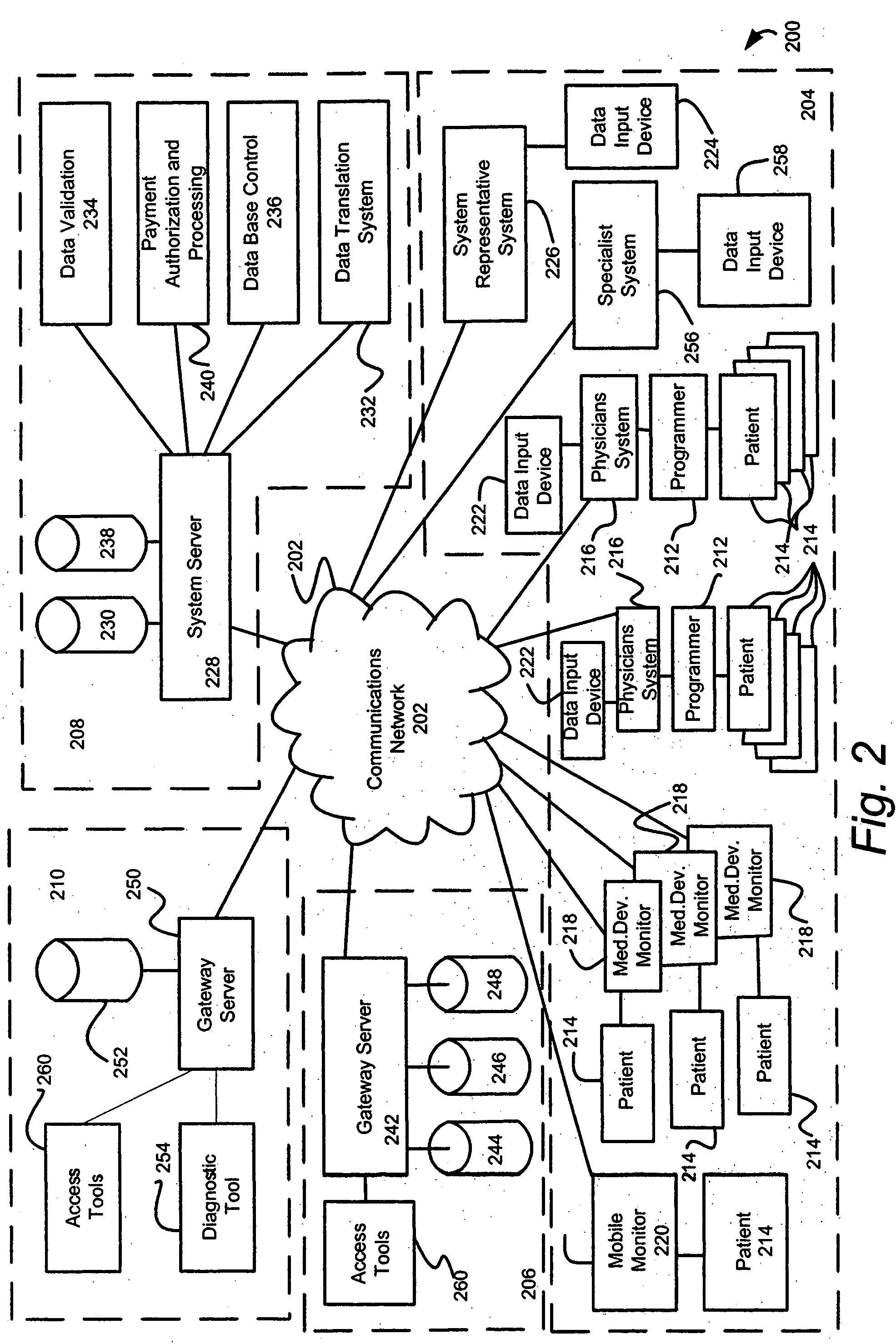
Communication System for Remote Patient Visits and Clinical Status Monitoring
InactiveUS20100128104A1Television conference systemsHospital data managementDiagnostic Radiology ModalityHospitalized patients
Disclosed embodiments include a telemedicine communication system designed to make possible “virtual” patient visits by approved visitors such as the spouse, family, and friends of the hospitalized patient having the appropriate digital certificate and visitation / communication permissions. According to one embodiment, the system enables relatives to visit hospitalized patients using audiovisual communication modalities selected according to the severity and health status of the patient. Additionally, the system provides functionality to enable approved relatives to follow the health status of the patient according to their permissions.
Owner:INNOVATEC
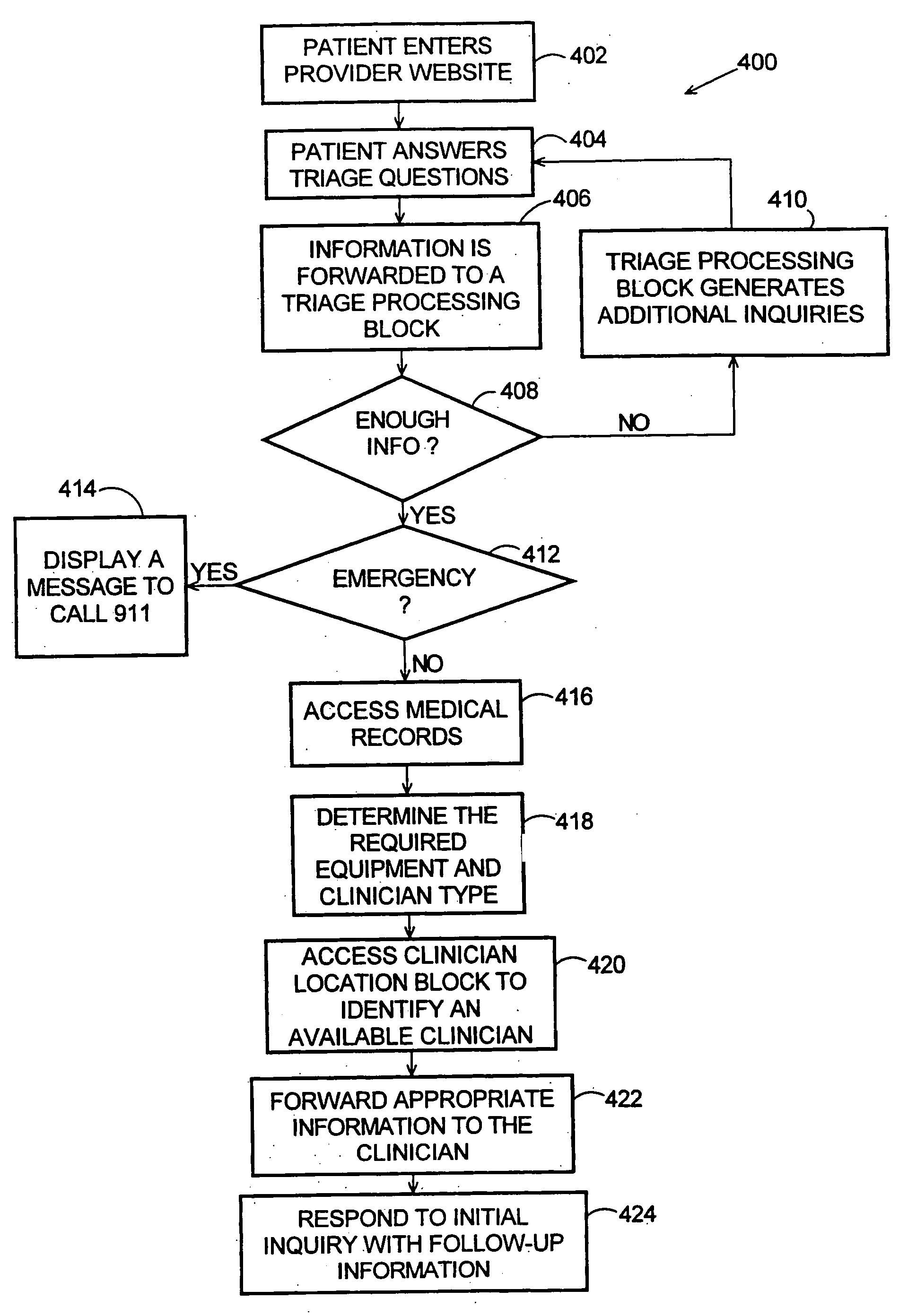
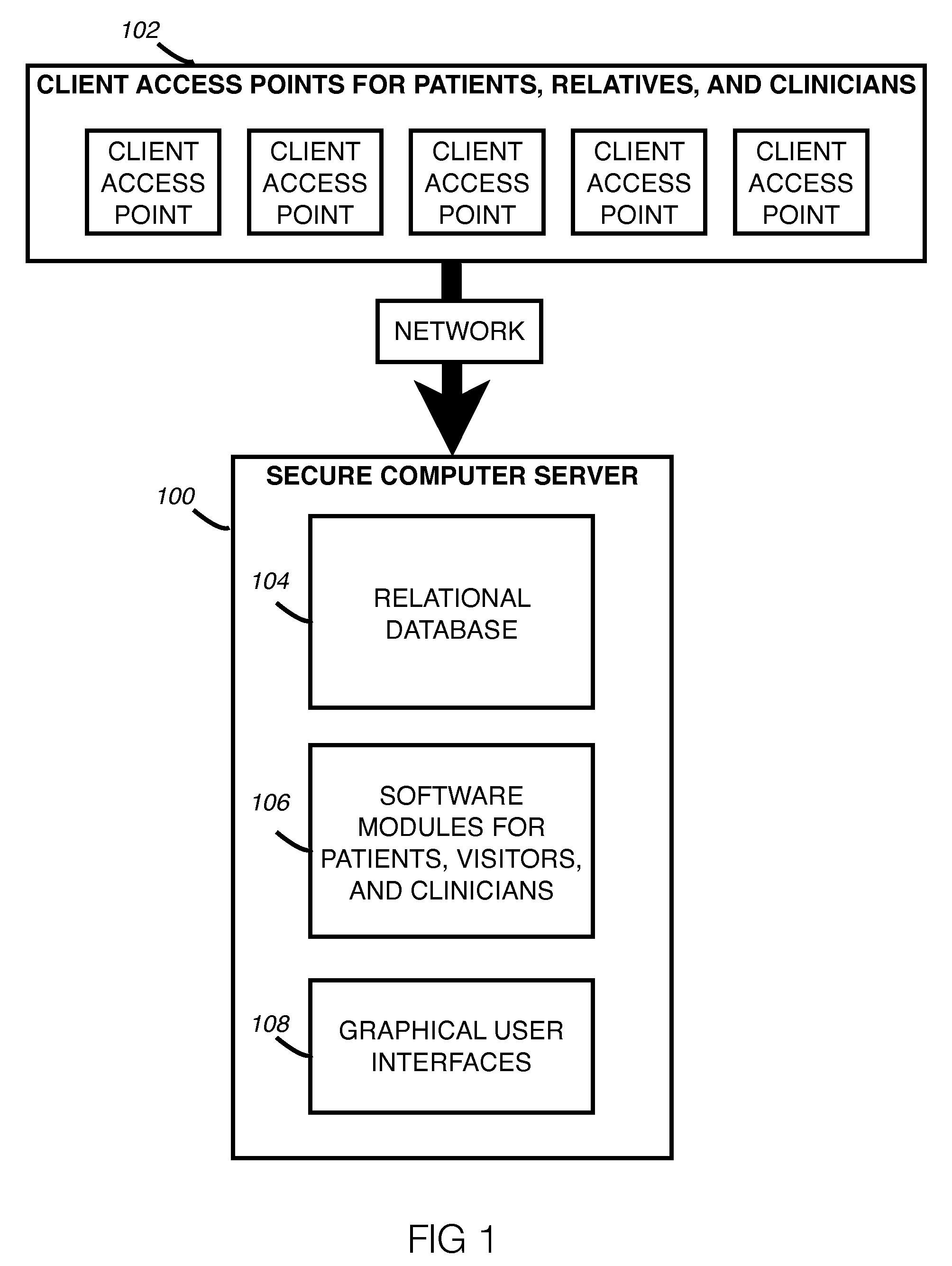
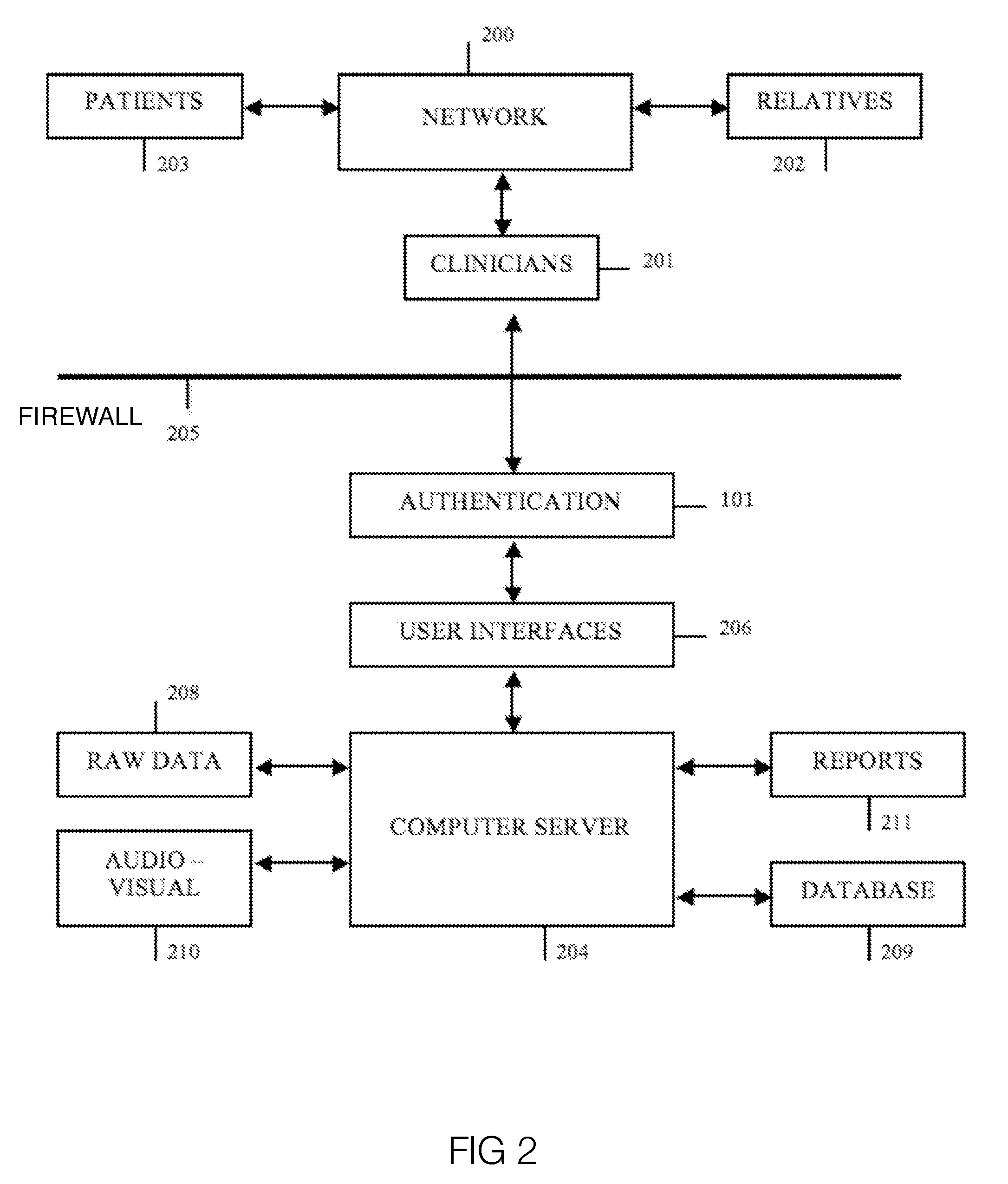
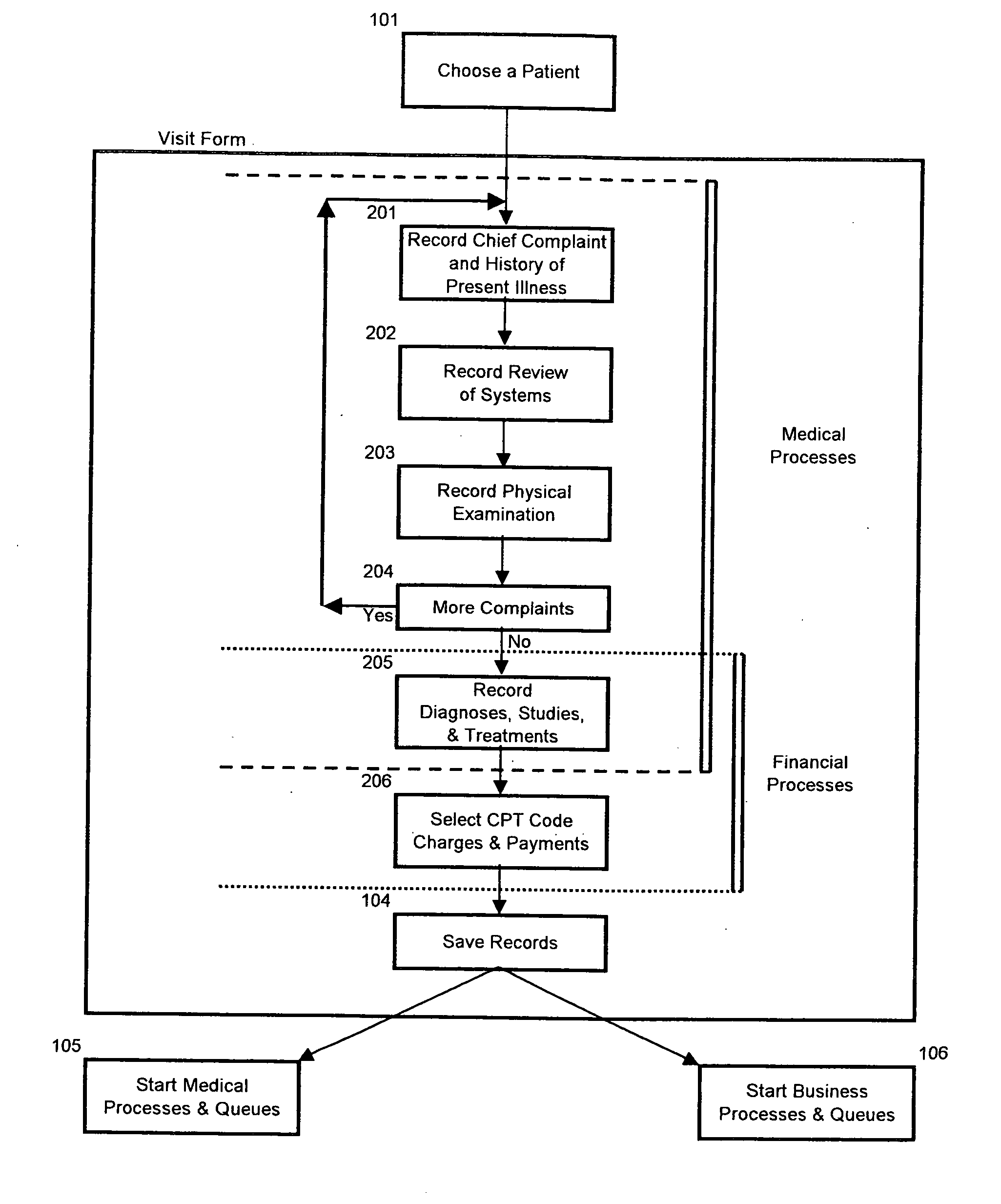
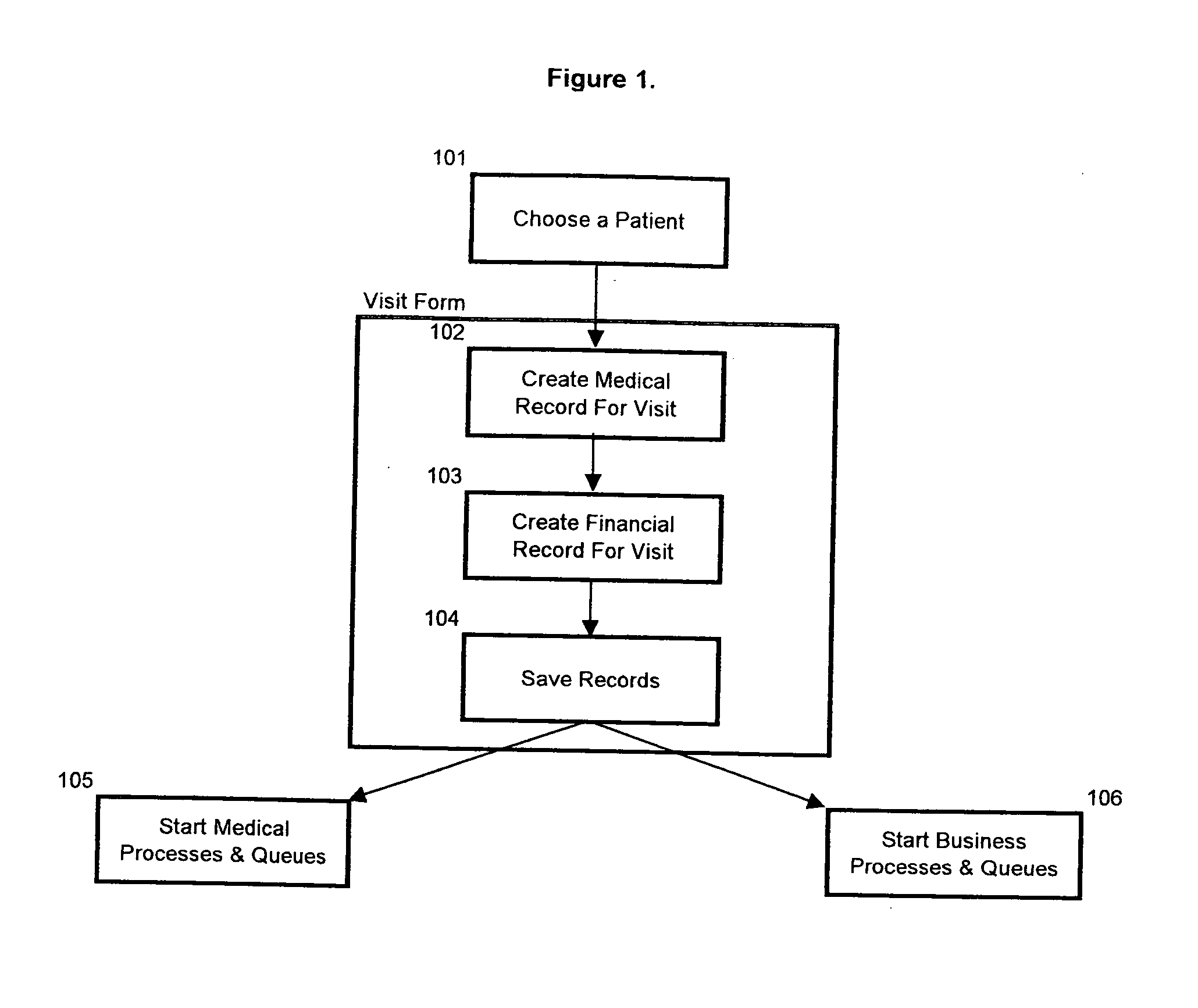
Method and system for creation of an integrated medical record via a communications computer network
InactiveUS20070294109A1Maximize efficiencyData processing applicationsDiagnostic recording/measuringMedical recordThe Internet
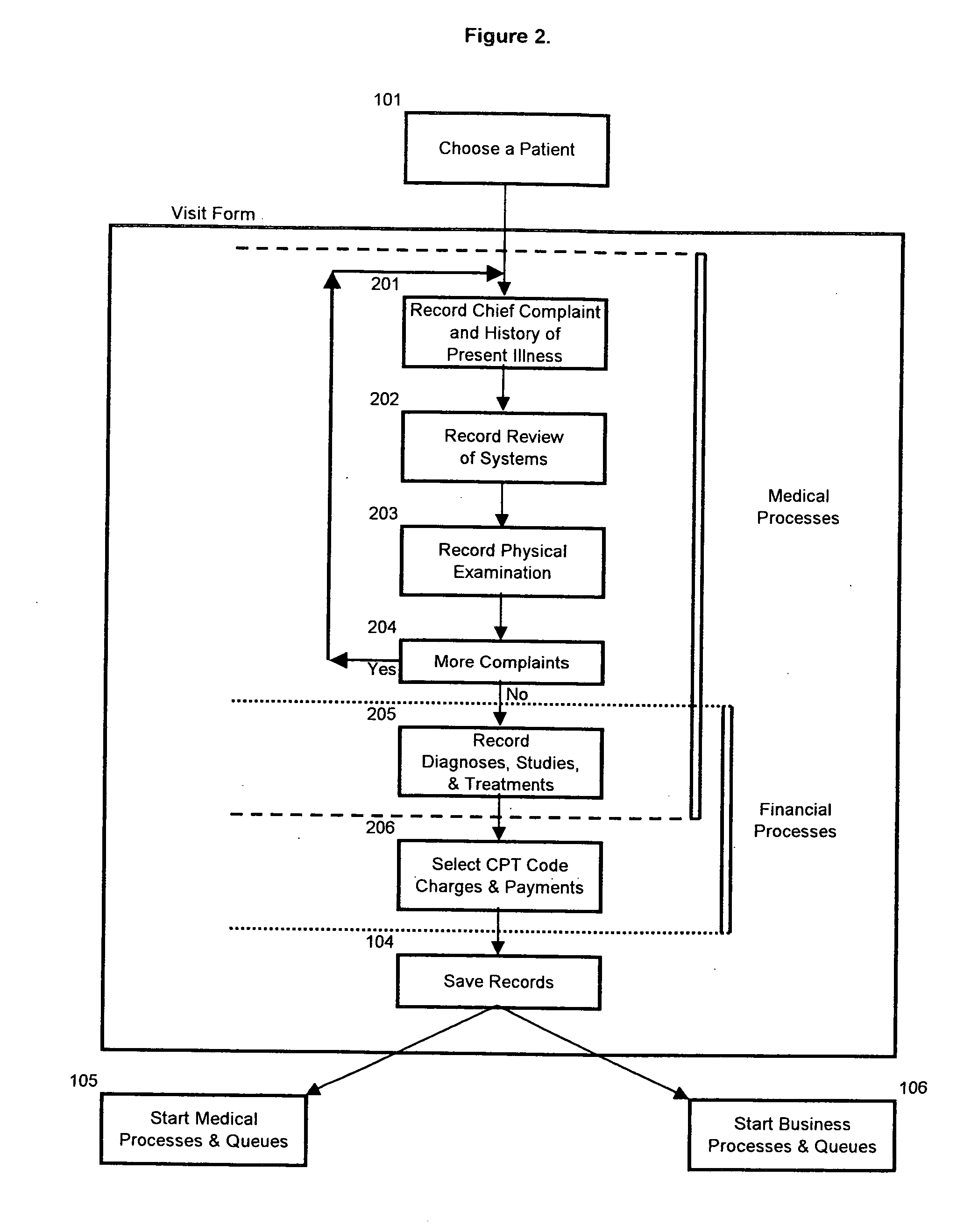
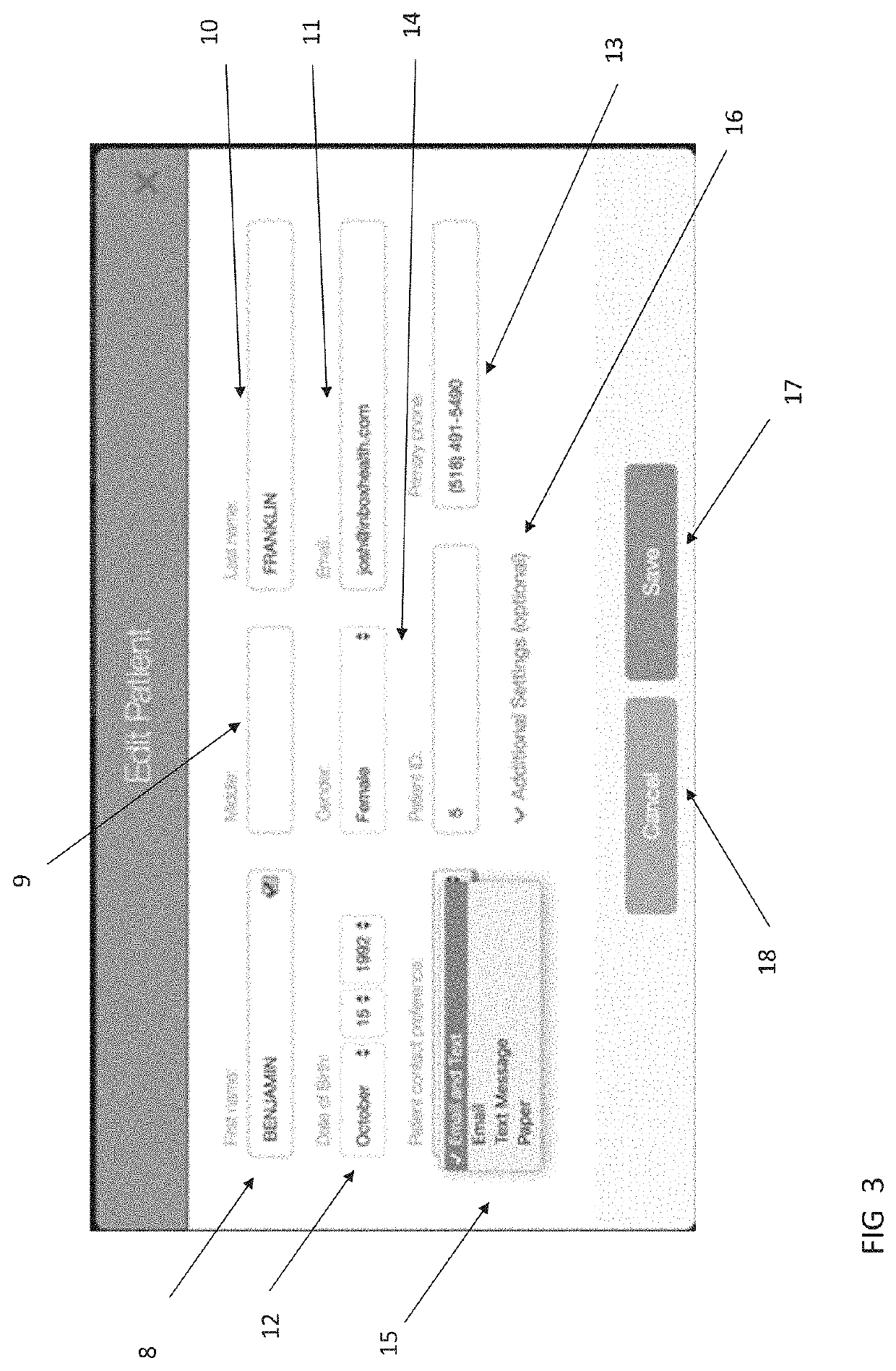
A method and system for single form creation of a patient visit medical record via the Internet that integrates with the financial system of the healthcare provider. A patient record generally has medical data and financial data. This system improves processing medical data, patient care, and financial information. First, the system provides a digital visit form, hosted on a server, and completed at the user's location. Second, the digital form contains lists, boxes, and buttons for data entry and work flow. Third, the system joins the diagnosis and the plan of the medical record including codes for the visit CPT, the diagnosis, procedure, and labs, prescription, pharmacy, referred provider, notes-and next appointment. This medical data then feeds into billing. Fourth, upon selecting the SAVE button, the system sends the medical and financial data to the server that updates the medical record, initiates medical and financial processes, and updates queue.
Owner:COSTELLO JOHN B
Methods, Devices, And Systems For Multi-Format Data Aggregation
InactiveUS20150213194A1Format structureSuperiority of formatData processing applicationsPatient personal data managementPoint of careData aggregator
Methods, devices, and systems for multi-format data aggregation, from disparate sources. According to an aspect, a method of populating a personal health record (PHR) with data culled from a plurality of images of a plurality of printed documents provided by a plurality of medical providers to a patient at a plurality of points of care after the patient visits the plurality of medical providers may be provided.
Owner:SYSTDICAL
Physicians' remote charge capture system
InactiveUS20050251417A1Facilitates organization and managementReduce laborFinanceOffice automationPatient managementComputer access
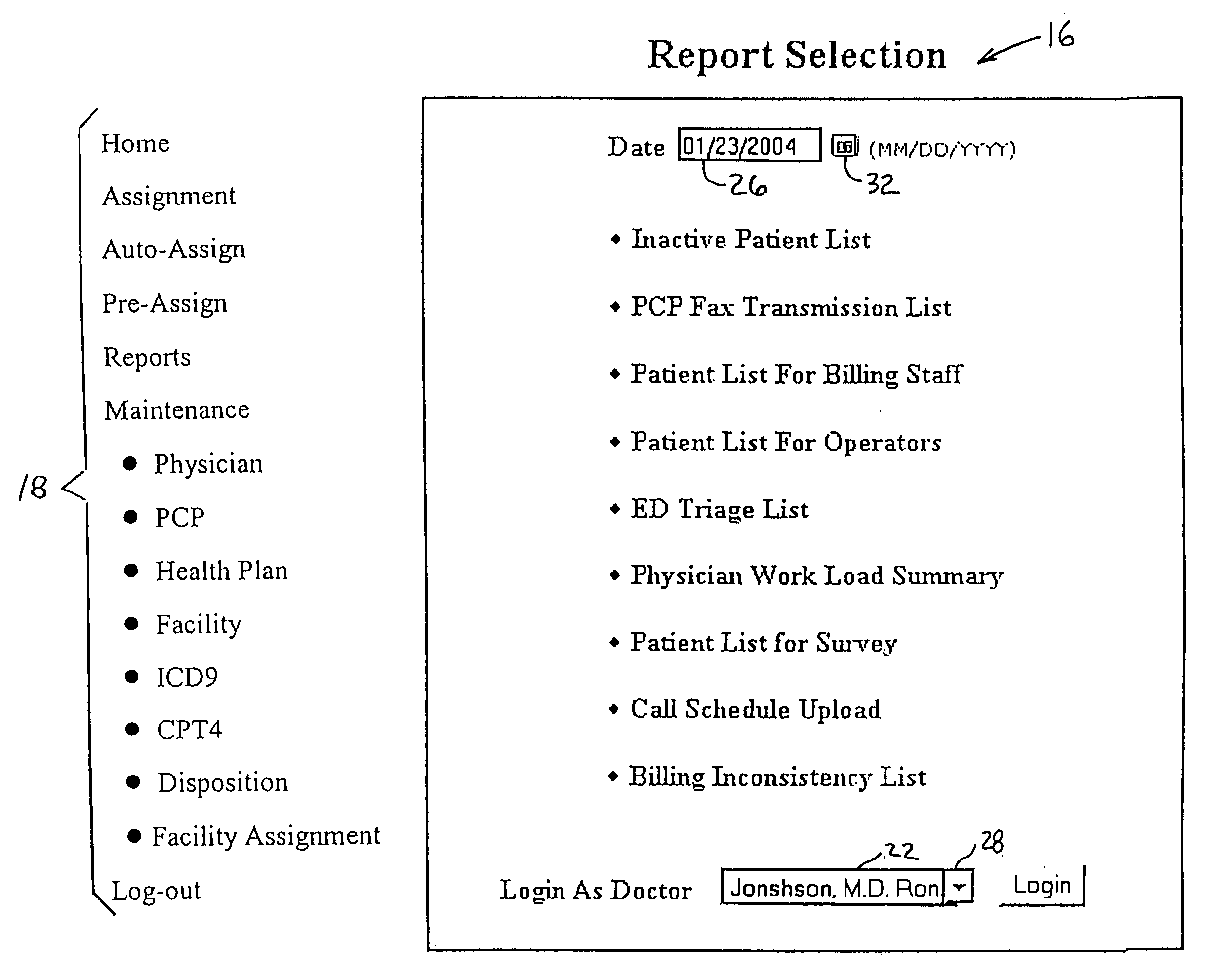
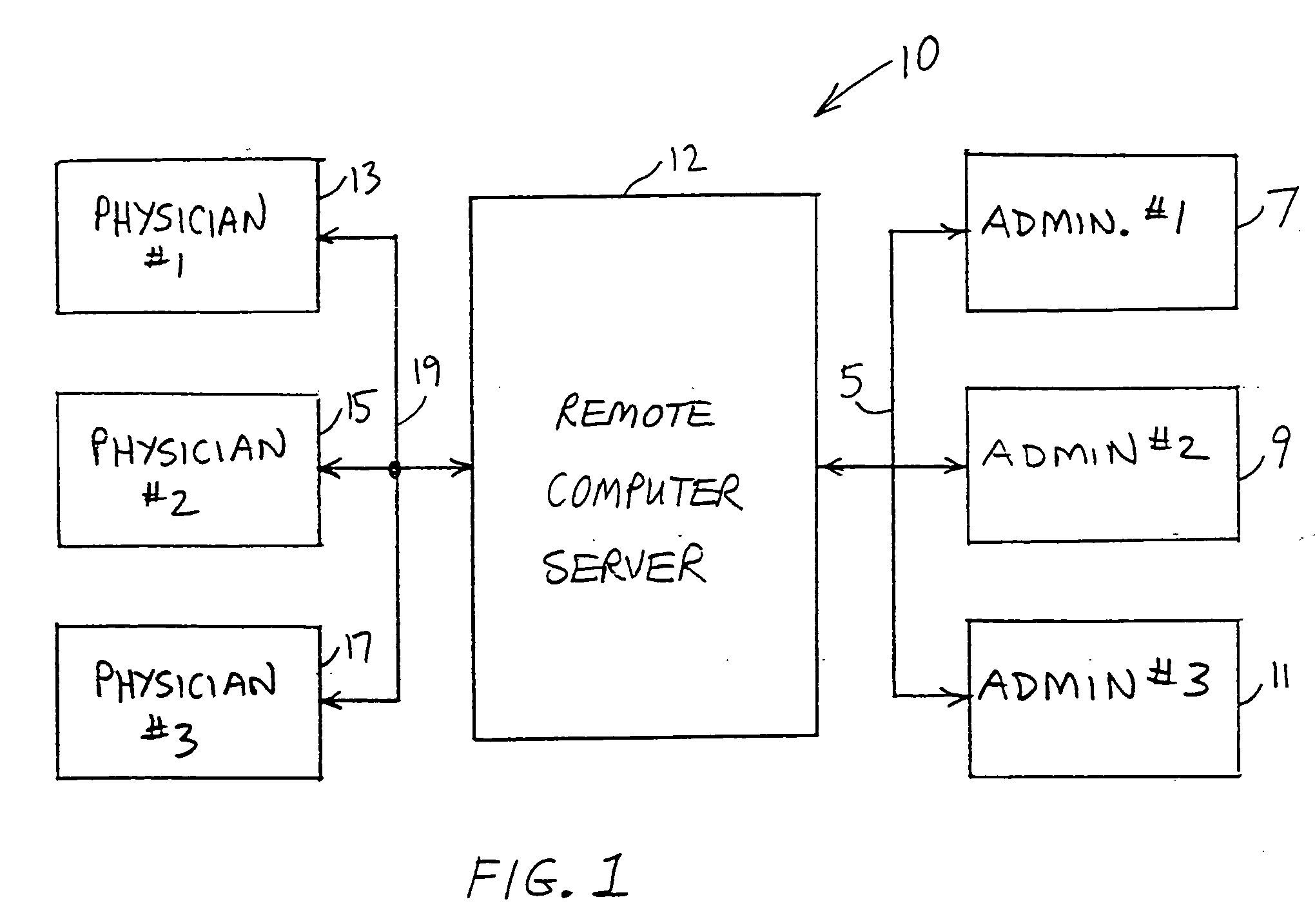
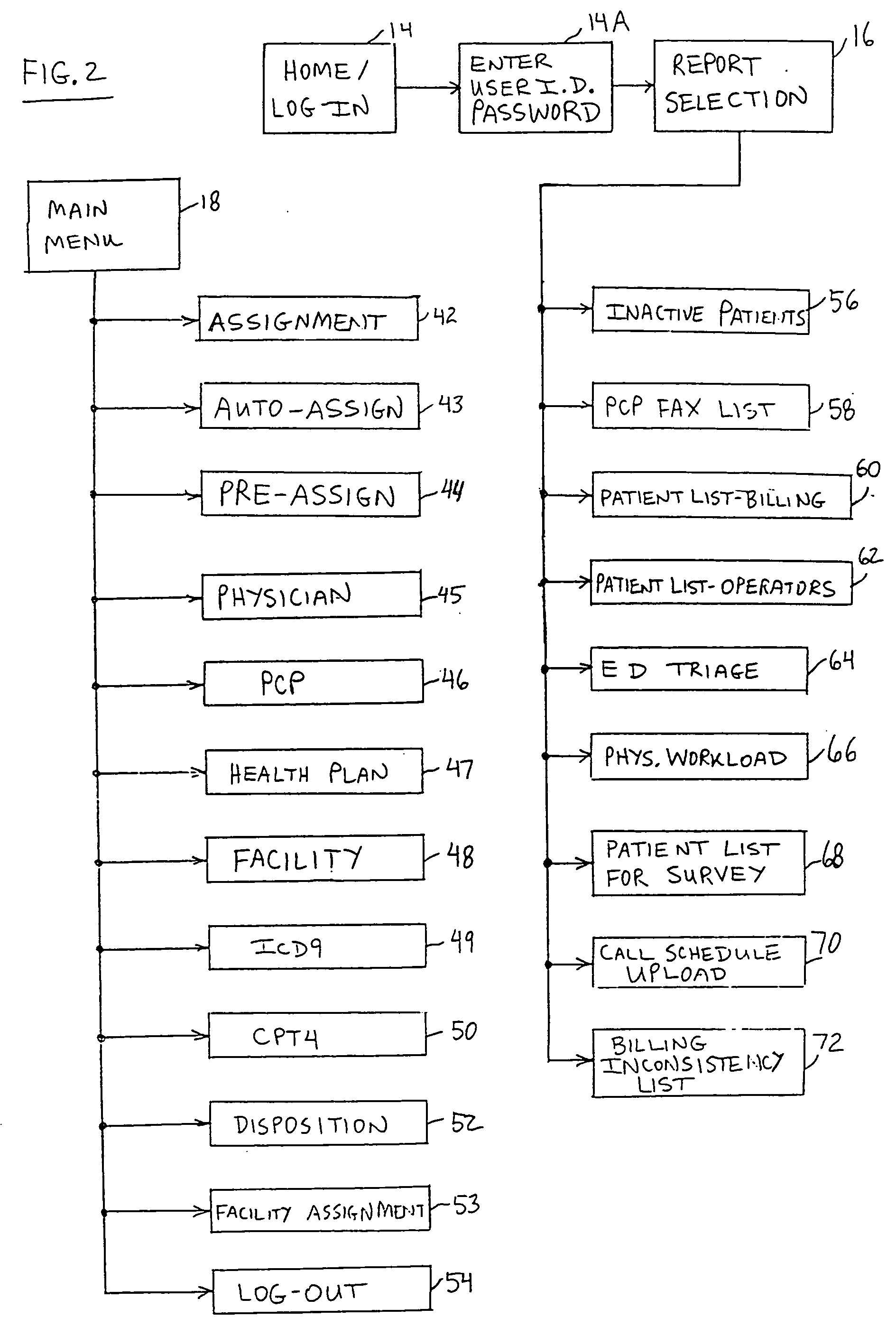
A patient management method for hospital-based physicians includes a remote computer server storing certain minimal information regarding patients of one or more physicians. The remote server is linked over a computer network to one or more physician-accessible computers remote from the computer server. Physicians can access such patient information over the network via the physician-accessible computer to view information regarding their patients. Following patient visits, the physician can update patient status information, such as diagnosis, treatment codes, and patient disposition, by entering such information into the server over the network. An administrator in the physician's office can also access the server over a computer network to capture updated patient status information for purposes of workload distribution and / or billing. The interposition of the server between the physician-accessible computer and the physician's office helps ensure compliance with the federal HIPAA Privacy Rule.
Owner:INDUS INVESTMENTS LTD PARTNERSHIP +1
Health information management system and method
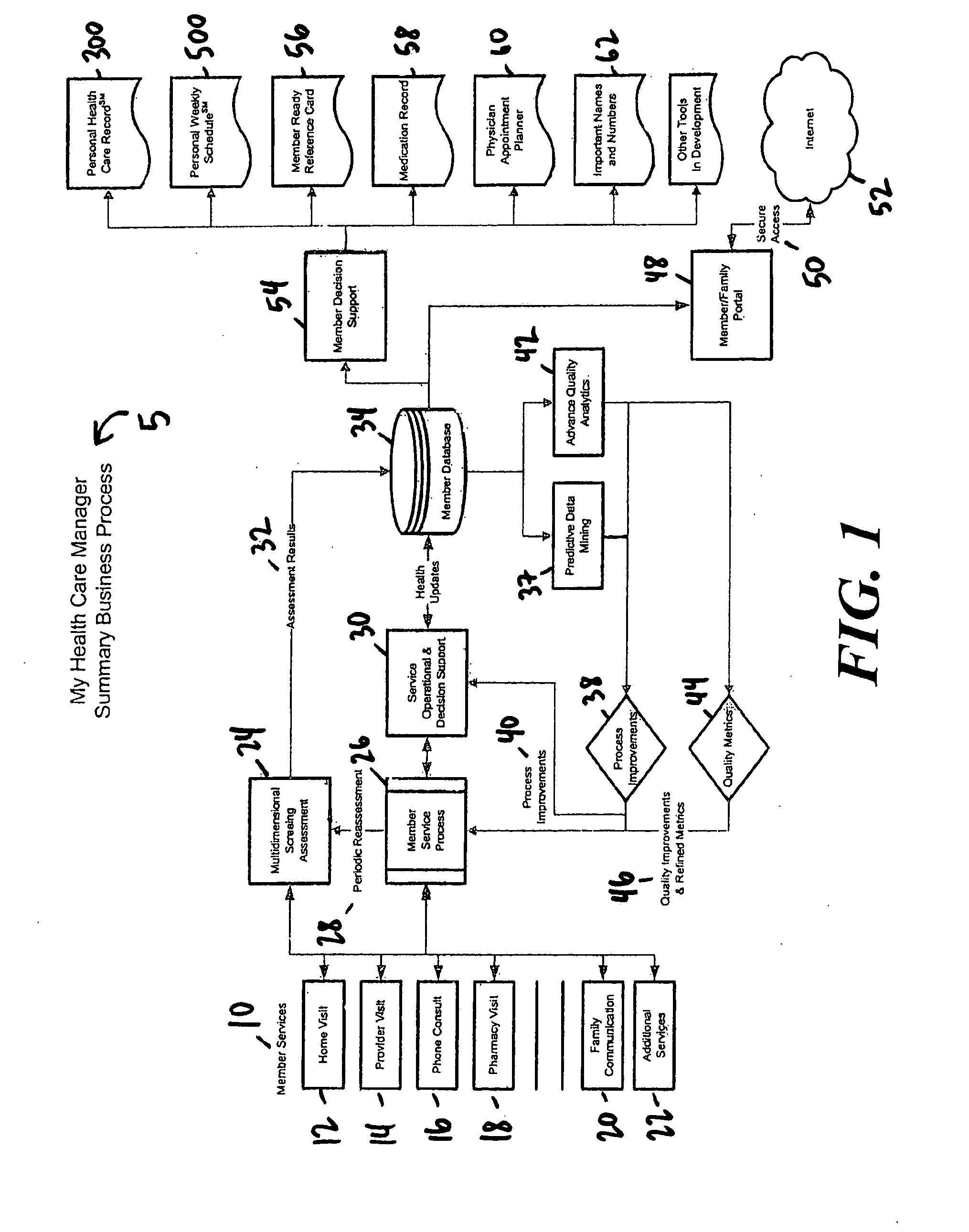
InactiveUS20080059242A1Increased direct interactionEasy to handleData processing applicationsHealth-index calculationPharmacyClinical psychology
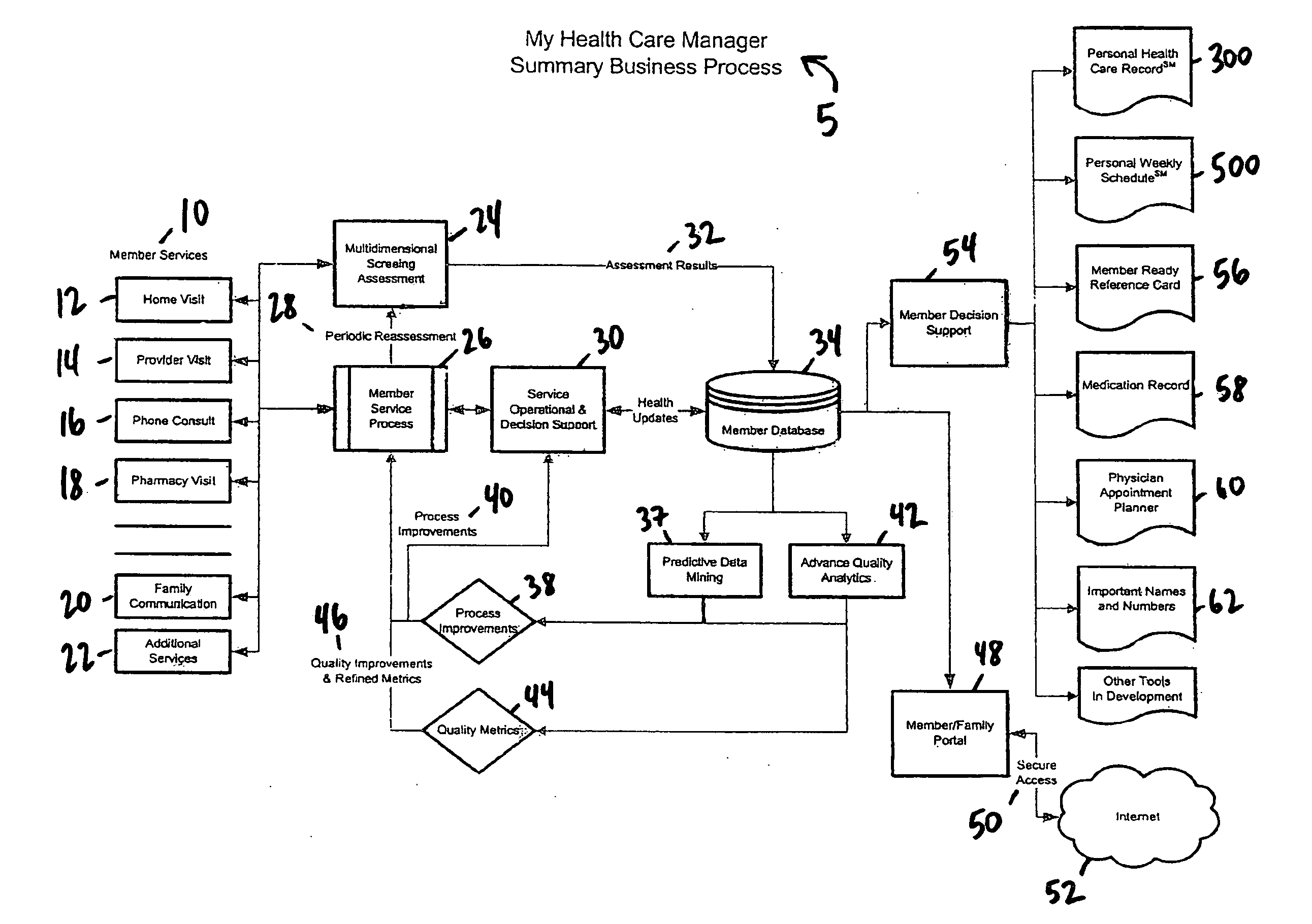
A health care management system and method. In one embodiment, a health care management system comprises a database capable of receiving data; a processor operably connected to the database, the processor having and executing a program and operational to receive patient data associated with a patient, said patient data obtained from at least one patient visit; perform a multidimensional screening assessment of the patient information; generate data from the multidimensional screening assessment; store in the database at least part of the patient data and at least part of the multidimensional screening assessment data; and generate at least one report from the stored data in the database. In another embodiment, the at least one member visit comprises one or more visits selected from the group consisting of a home visit, a provider visit, a phone consult, a pharmacy visit, and a family communication.
Owner:MY HEALTH CARE MANAGER INC
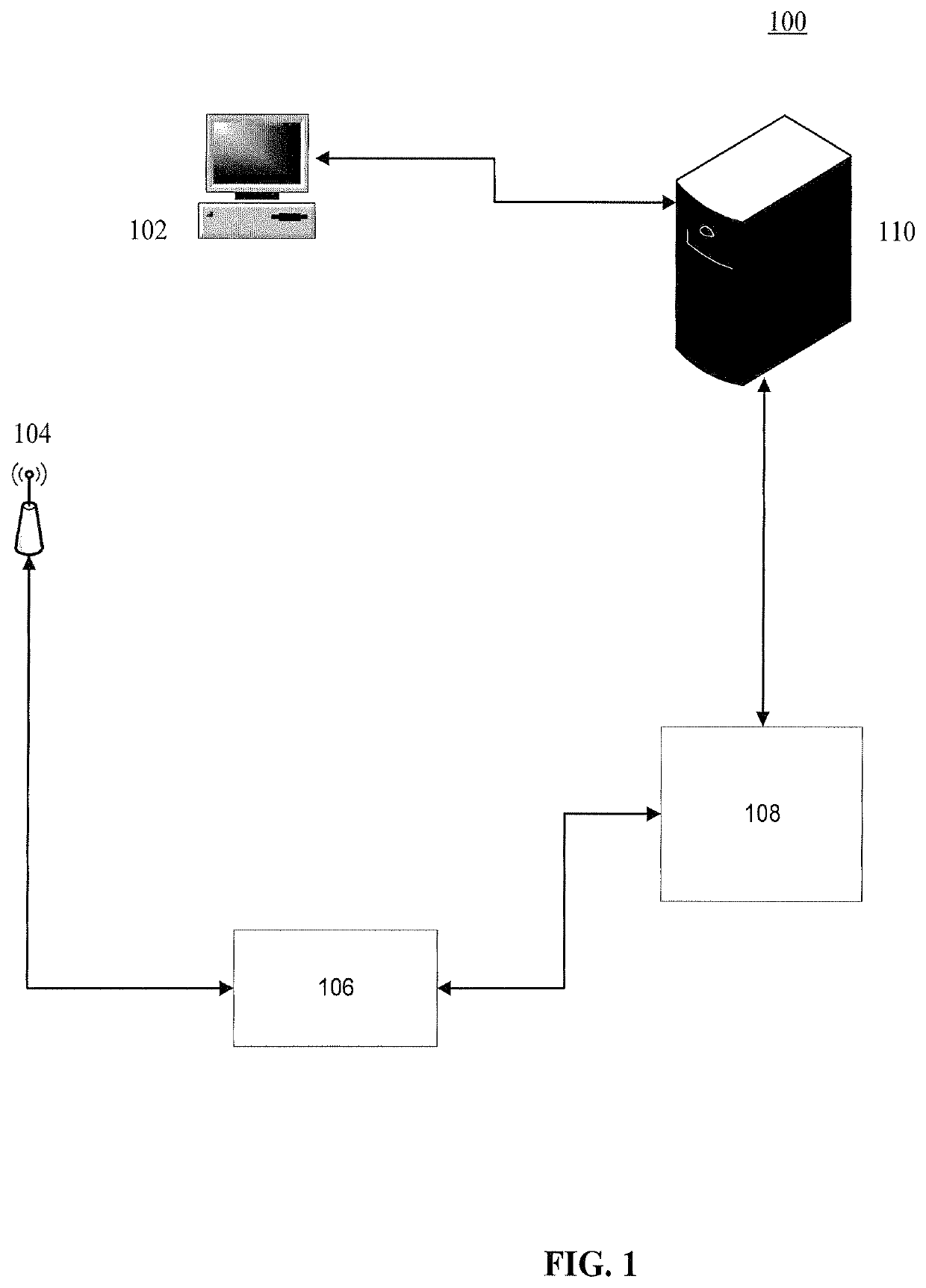
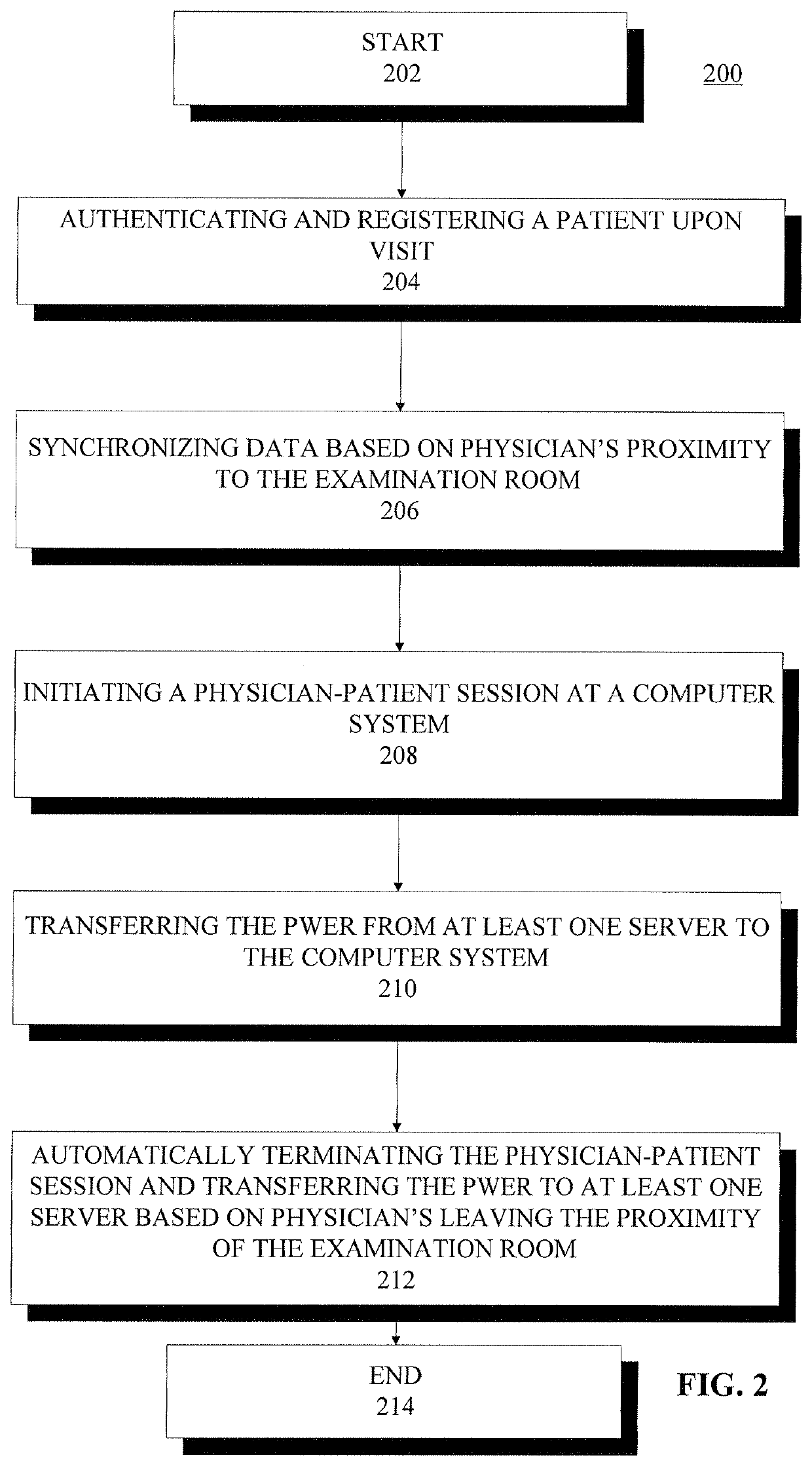
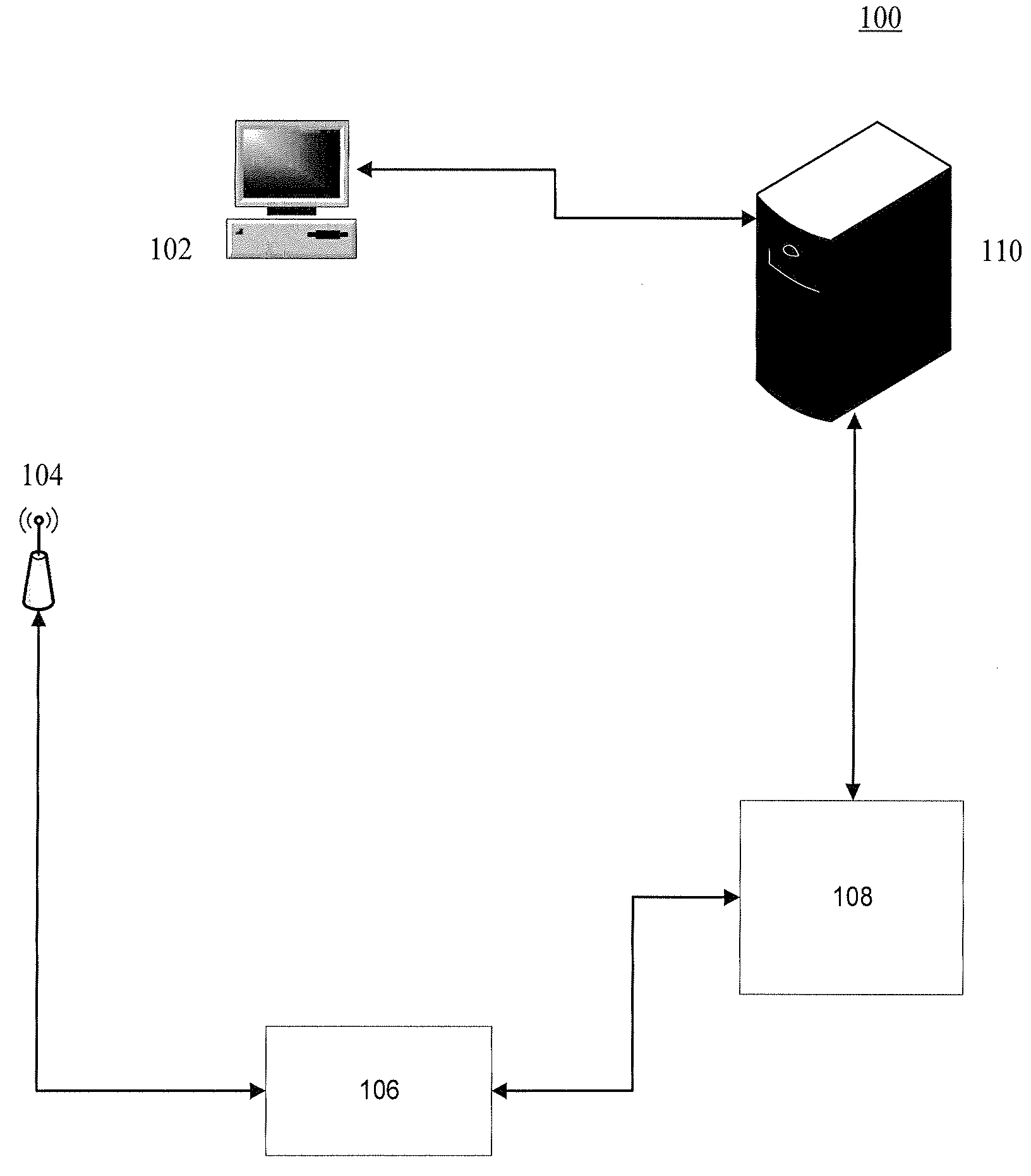
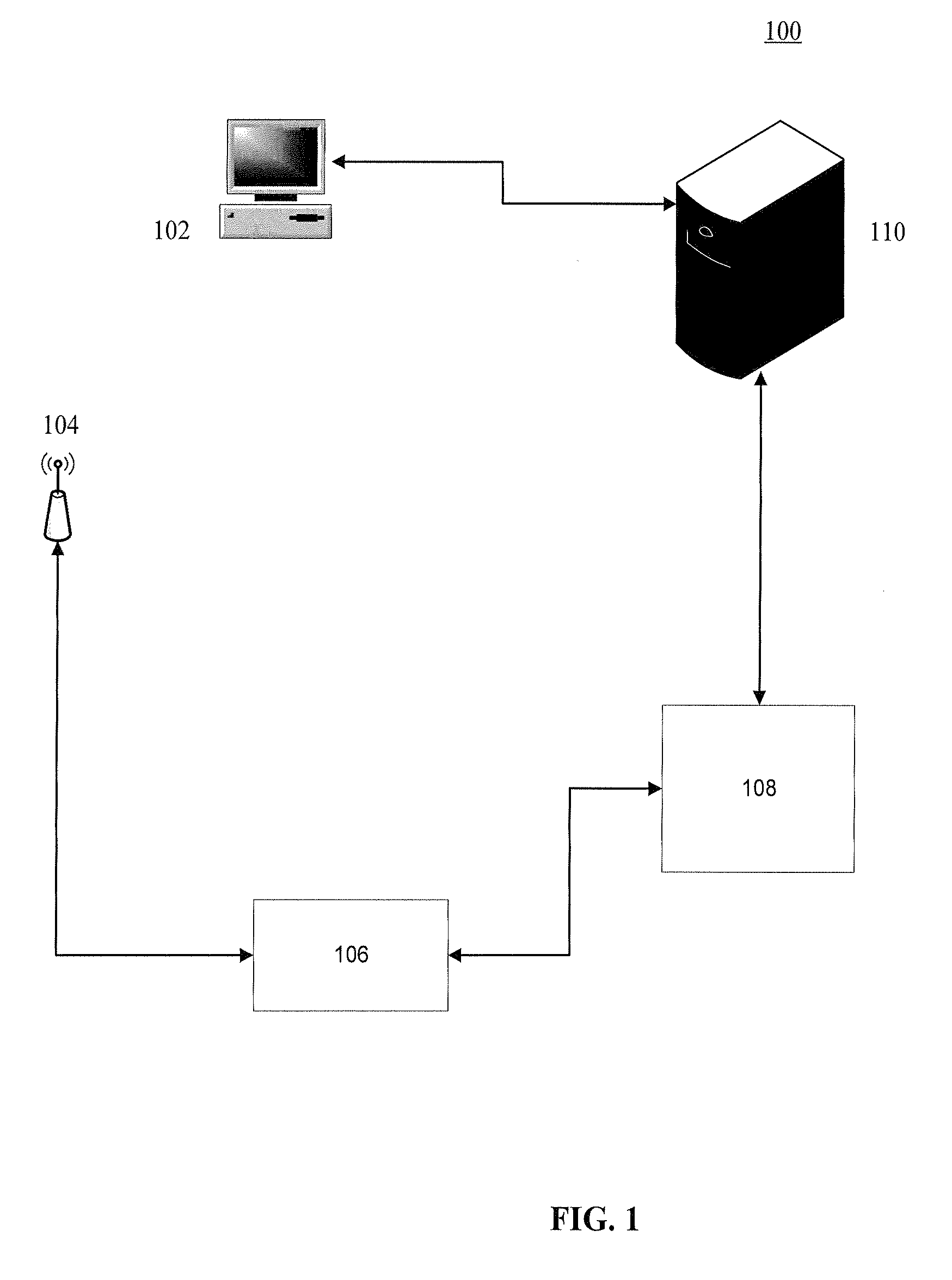
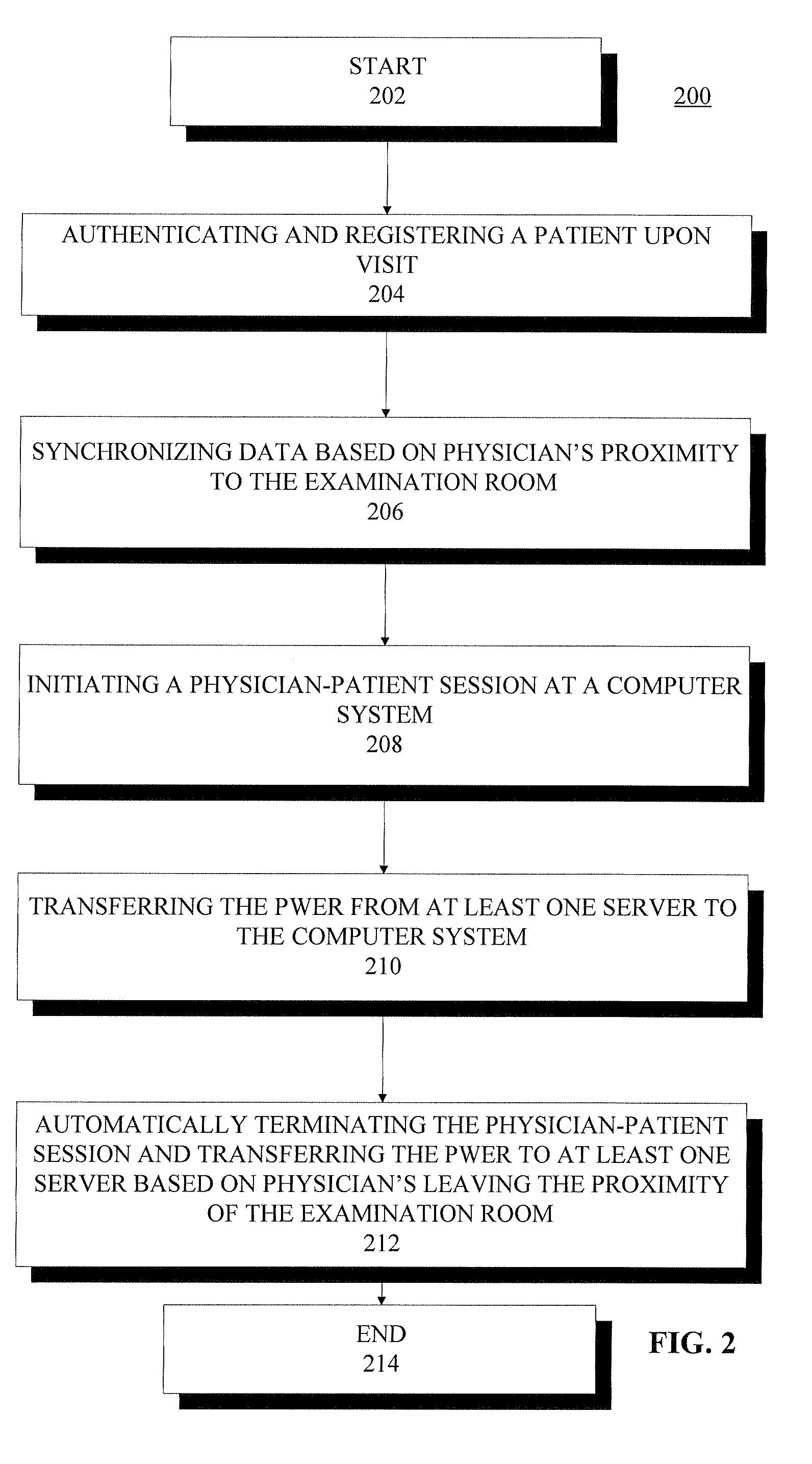
System and method for making patient records follow a physician
A computer-based system for providing physicians automatic, secure access to patient records at the time a patient visits and consults a physician. The system can include one or more computing devices configured to process and display data. The system can also include one or more emitting devices carried by physicians for identifying each particular physician and one or more scanning devices configured to detect and communicatively link to the one or more emitting devices based on the proximity of the physician to an examination room. Additionally, the system can include a manager module communicatively linked to the one or more scanning devices and configured to manage the signal data from the one or more scanning devices. Furthermore, the system can also include one or more servers communicatively linked with the one or more computing devices, one or more scanning devices, and manager module, the one or more servers configured to authenticate and register a particular patient upon a visit to a hospital or office, synchronize the particular physician identifier, patient identifier, personal wellness electronic record (PWER), and examination room identifier based upon physician's proximity to an examination room, initiate a physician-patient session at the at least one computing device at the examination room, transfer the PWER to the at least one computing device at the examination room, and automatically terminate the physician-patient session and receive the PWER based upon the physician leaving the proximity of the examination room.
Owner:THE QUANTUM GROUP +1
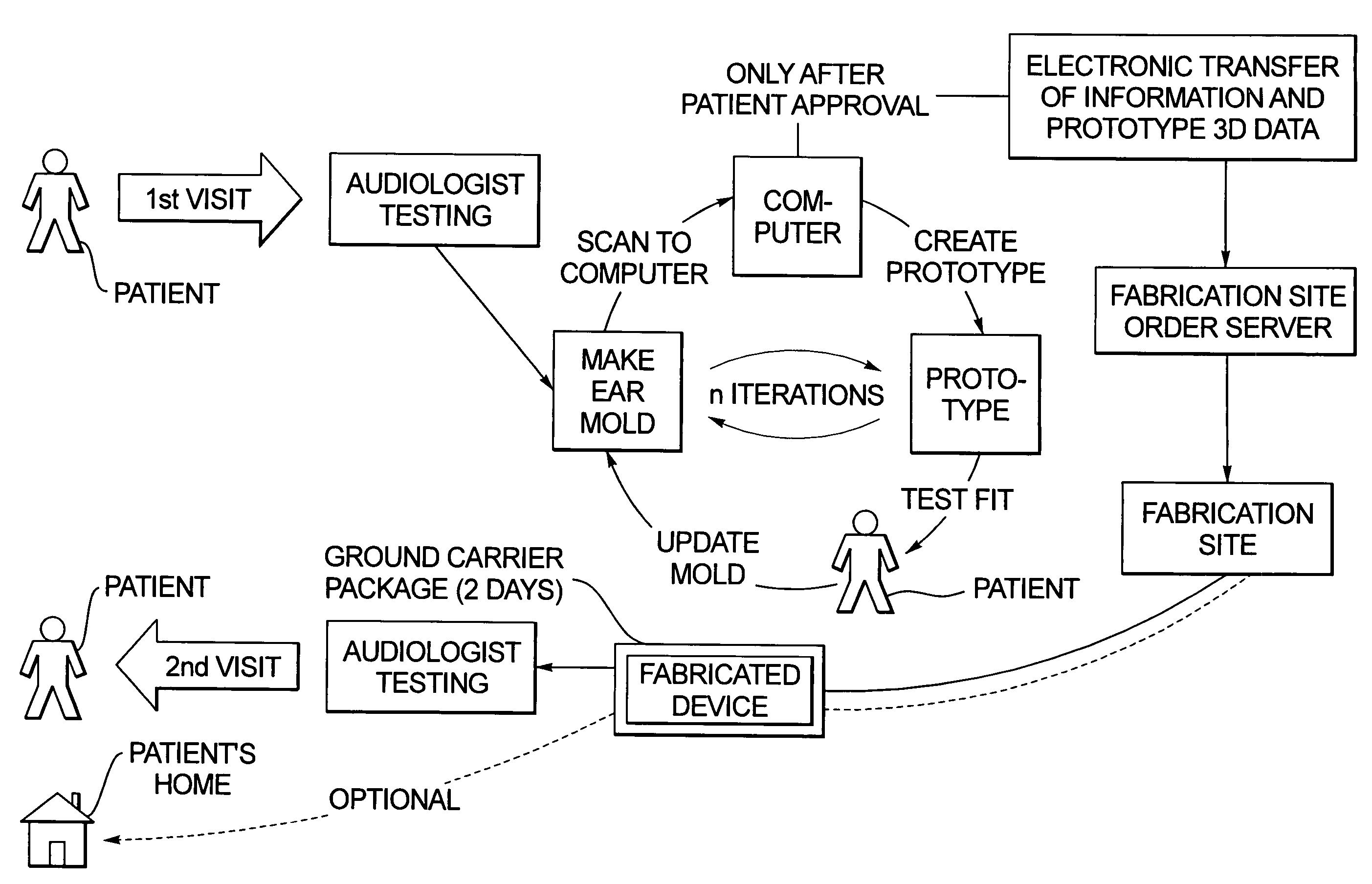
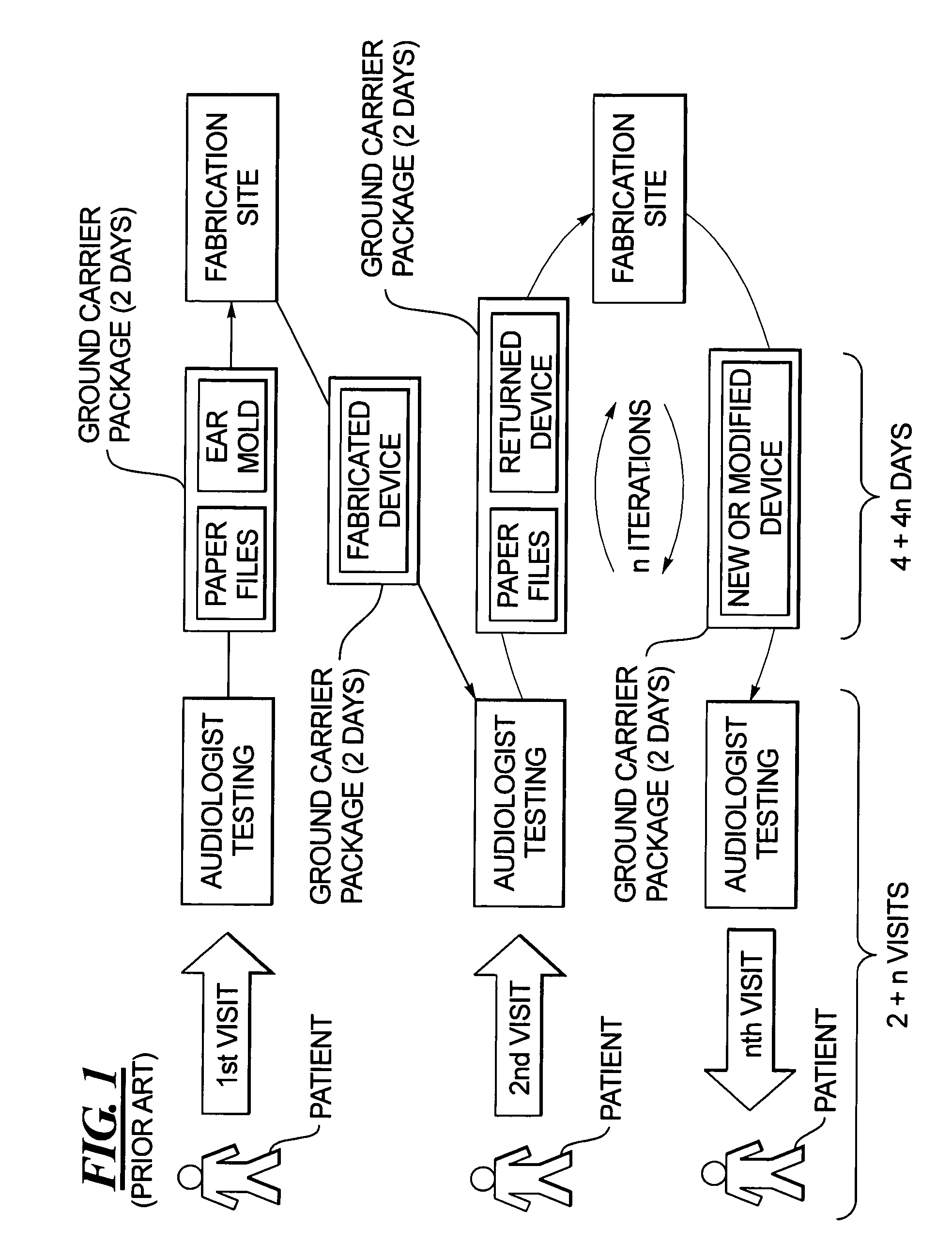
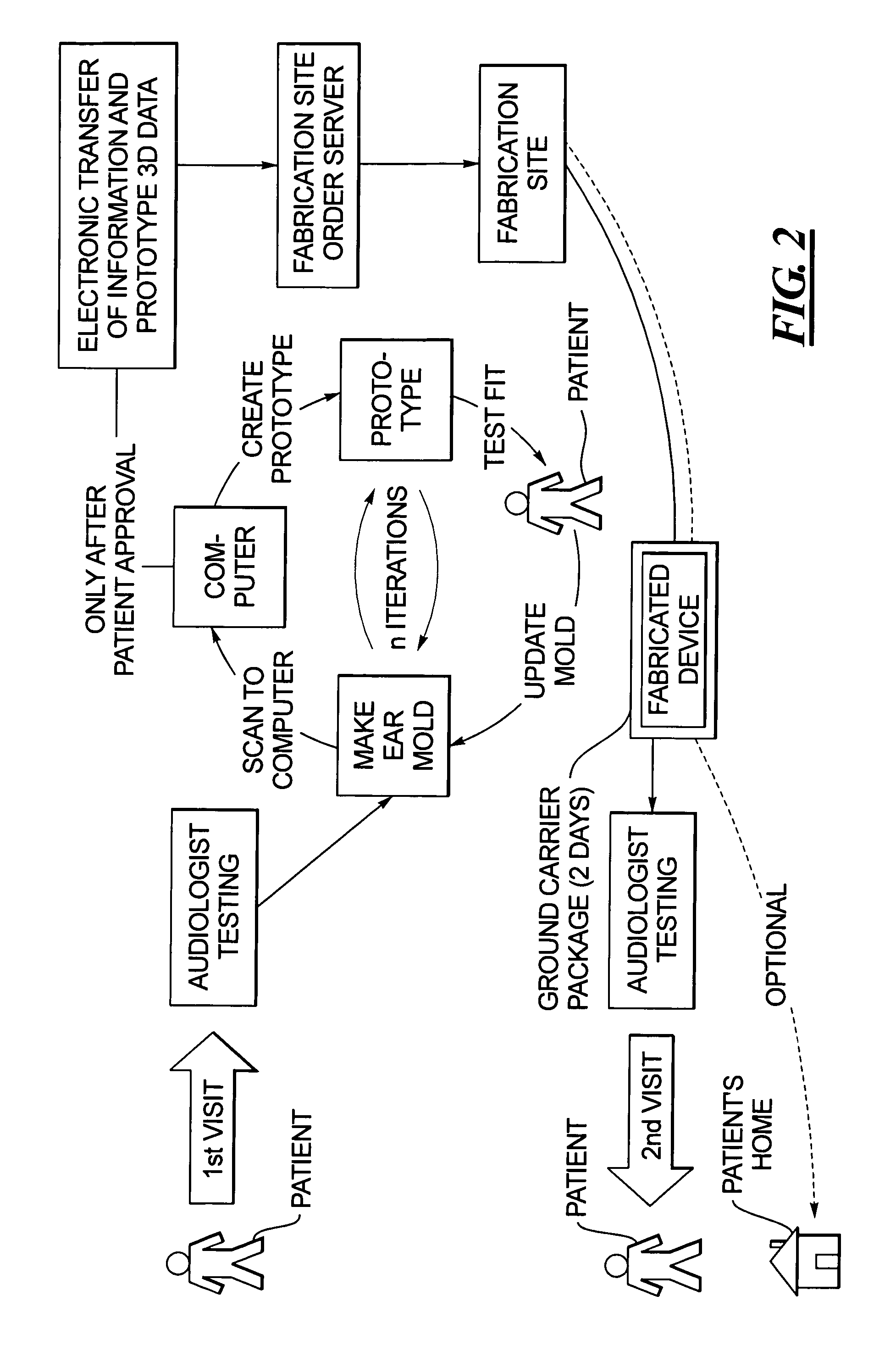
Method for fabricating a hearing aid shell and mold incorporating test fitting by the user
InactiveUS7467022B2Avoid delayFabrication avoidedHearing aid design aspectsSpecial data processing applicationsData setAudiologist
In a method for fabricating a hearing aid device, a patient visits an audiologist at a dispenser location, and is examined to determine electronic settings for a hearing aid to correct the patient's hearing impairment. At the same visit, an ear mold of the patient is obtained, which is scanned to produce a three-dimensional data set, from which a prototype is produced from the hearing aid data set, that does not contain any electronic components, and the prototype is test fitted with the patient. By interaction between the patient and the audiologist, the prototype is modified as needed. When the prototype is acknowledged by the patient as being a comfortable fit, the three-dimensional data that were used to create the acceptable prototype are electronically transmitted to a fabrication site, at which the hearing device is manufactured therefrom. The hearing device is then sent to a location at which it is available to the patient.
Owner:SIEMENS AG
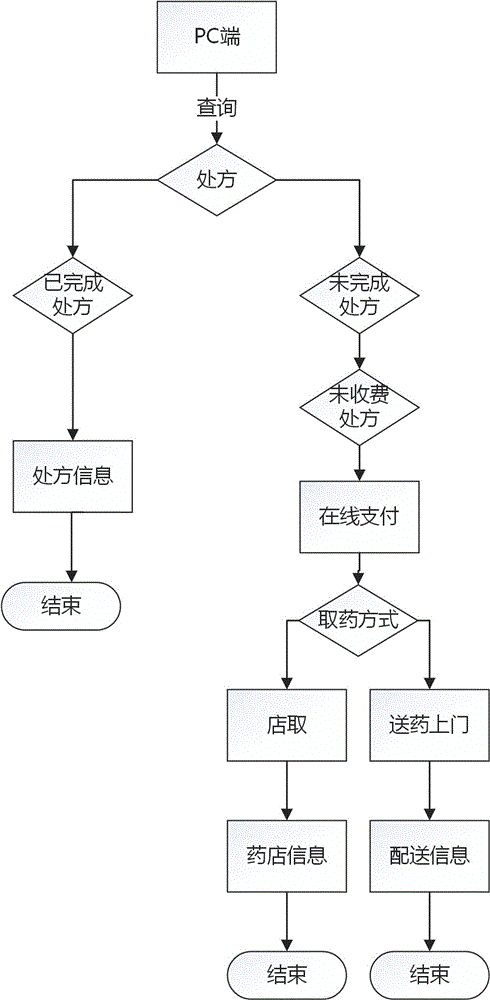
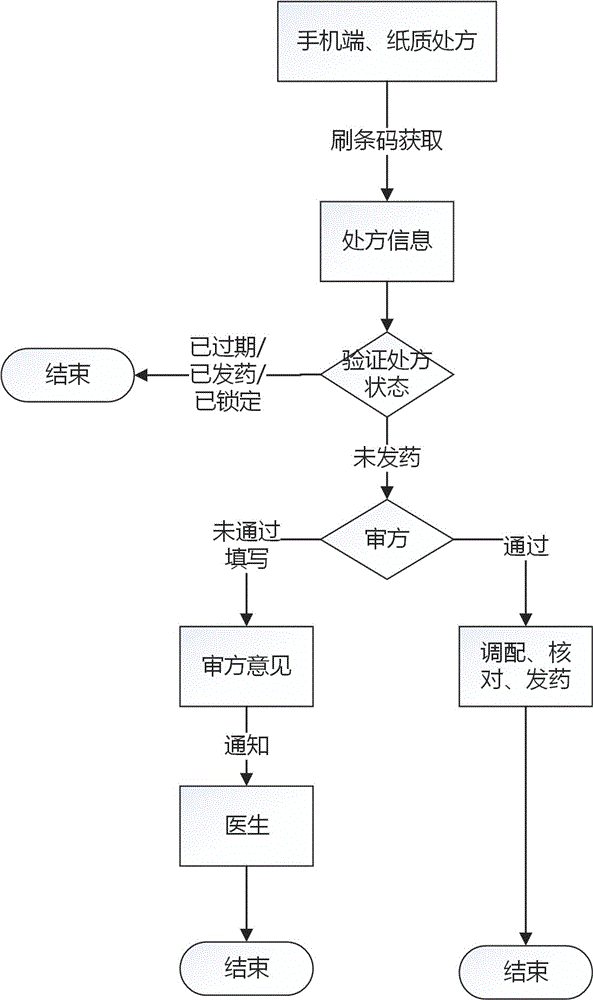
Internet medical multidimensional network electronic prescription information processing and identifying method
InactiveCN106803020AUniqueness guaranteedEnsure consistencyTelemedicineComputer-assisted treatment prescription/deliveryInformation processingThe Internet
The invention relates to an internet medical multidimensional network electronic prescription information processing and identifying method. According to the method, <<prescription administrant methods>> serve as standards, internet medical services are matched by the aid of digital secret key and barcode recognition technology, prescriptions drawn by doctors on internets are converted into a multidimensional network electronic prescription system with secret key protection and code identification, the multidimensional network electronic prescription system comprises a doctor terminal, a patient terminal and a drugstore terminal, the doctor party, the patient party and the drugstore party perform corresponding operations through the multidimensional network electronic prescription system, and the multidimensional network electronic prescription system can be connected with a doctor diagnosis and treatment information system, a patient visiting system and a drug selling system. The method can achieve uniqueness and authenticity of electronic prescriptions.
Owner:福州互医信息科技有限公司
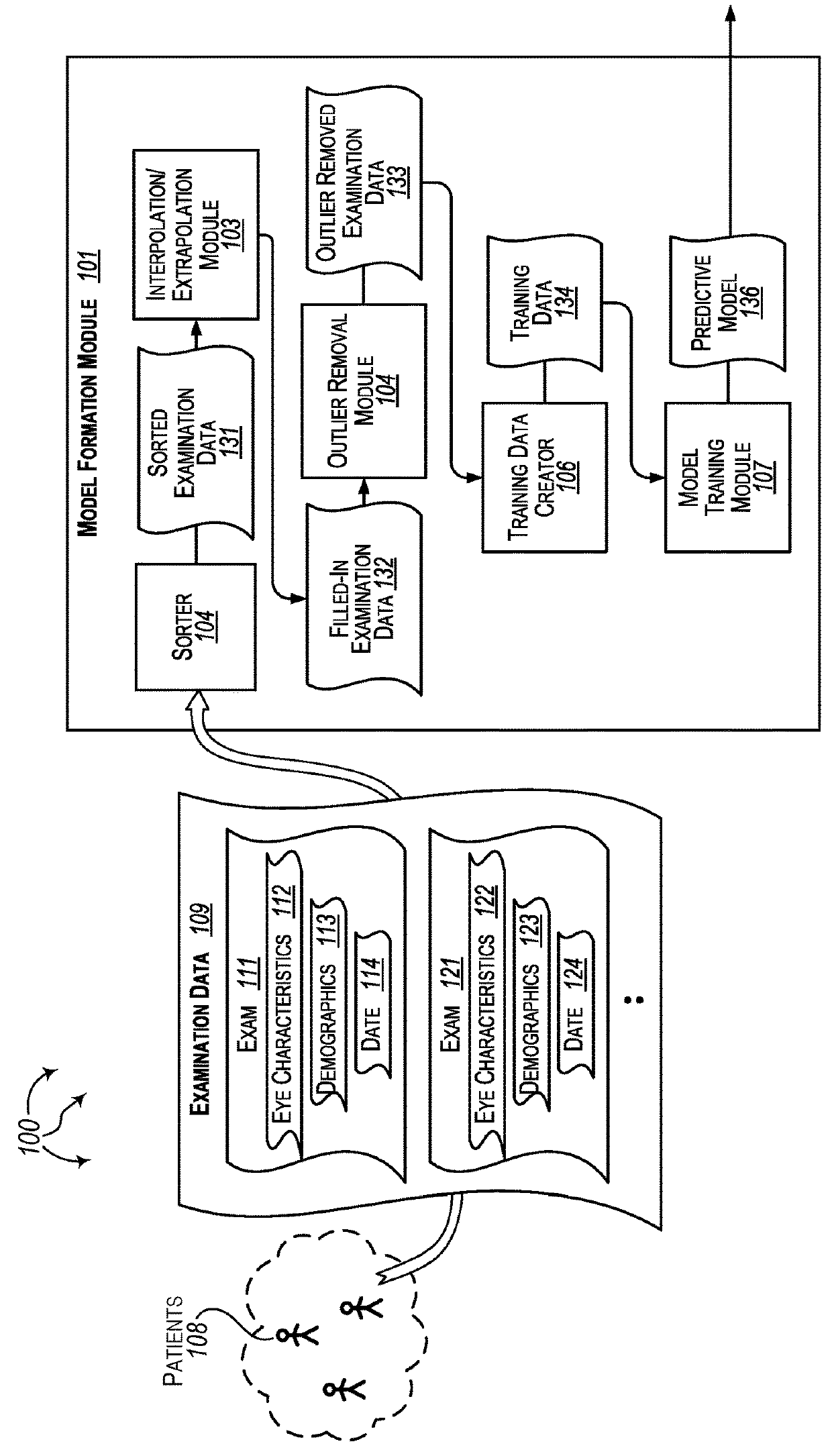
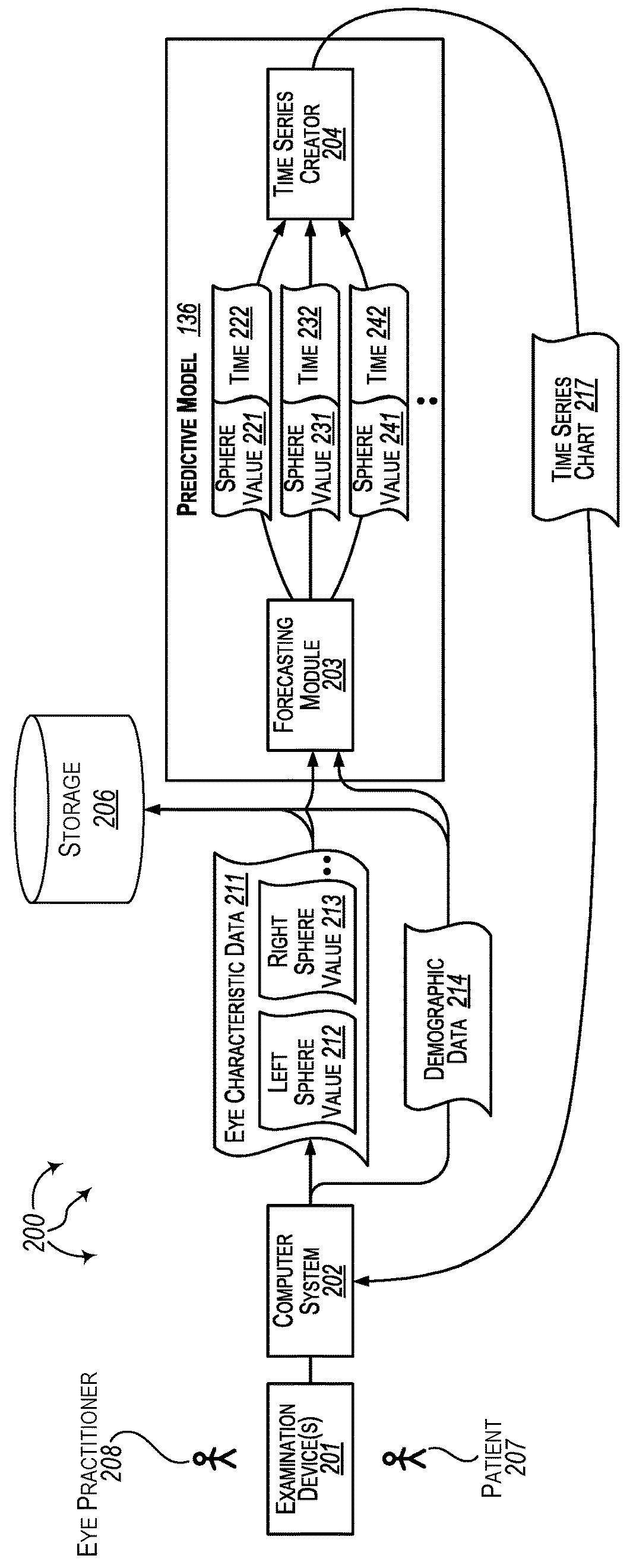
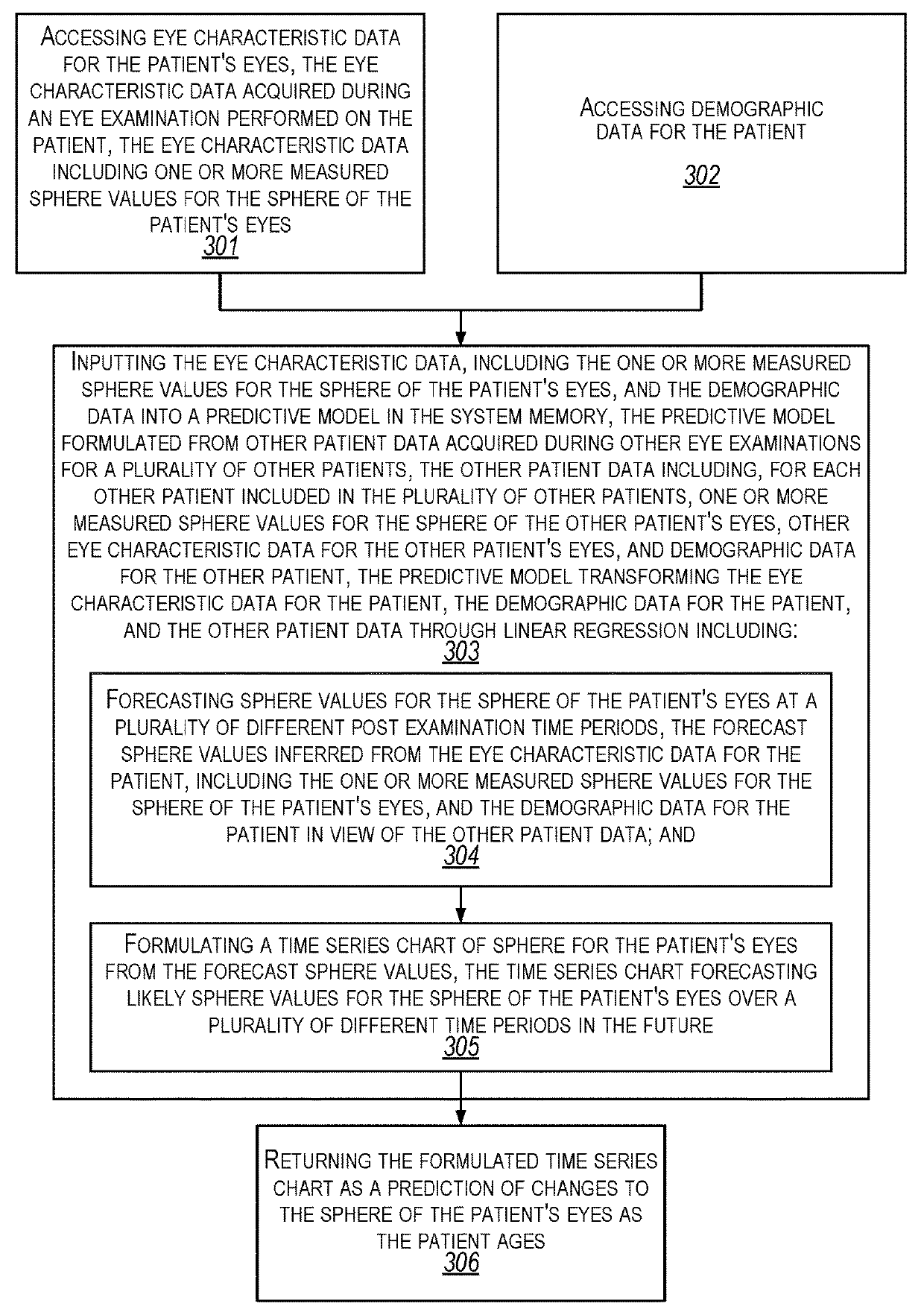
Forecasting eye condition progression for eye patients
Aspects extend to methods, systems, and computer program products for forecasting eye condition progression for eye patients. When a patient visits an eye practitioner, the patient (or when appropriate their guardian) may be interested in the current eye condition as well as a prediction of eye condition progression in the future and / or as the patient ages. Aspects of the invention can be used to predict the progress of an eye condition for a patient (e.g., a child) at a number of different post-examination times after an examination. Predicting the progress of an eye condition for a patient over time can be used to assist the eye practitioner in tailoring a treatment plan and / or tailoring a subsequent examination schedule for the patient.
Owner:MICROSOFT TECH LICENSING LLC
Personal respiratory protection system
A respiratory protection system which is worn by a passenger during a flight in air plane, by a patient visiting hospital or doctors office, a nurse working with a patient during hospital stay, or the like. The system includes a relatively light weight, substantially rigid, headgear structure, containing an air disinfection chamber, a fan means, a filter means, an ultraviolet air disinfection means, an air blanket origination means and an energizing means.
Owner:GLAZMAN MARK
System for Providing an Overview of Patient Medical Condition
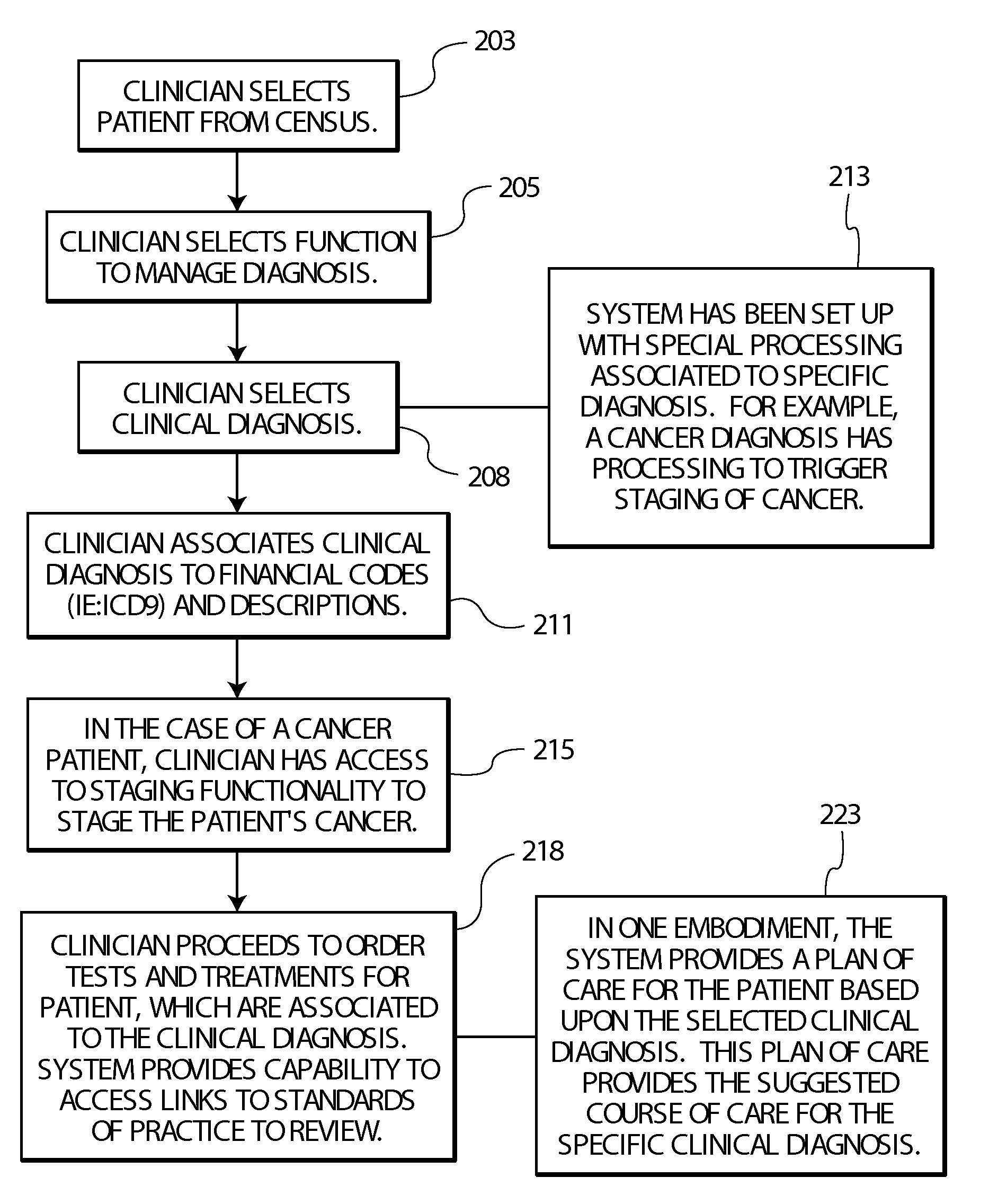
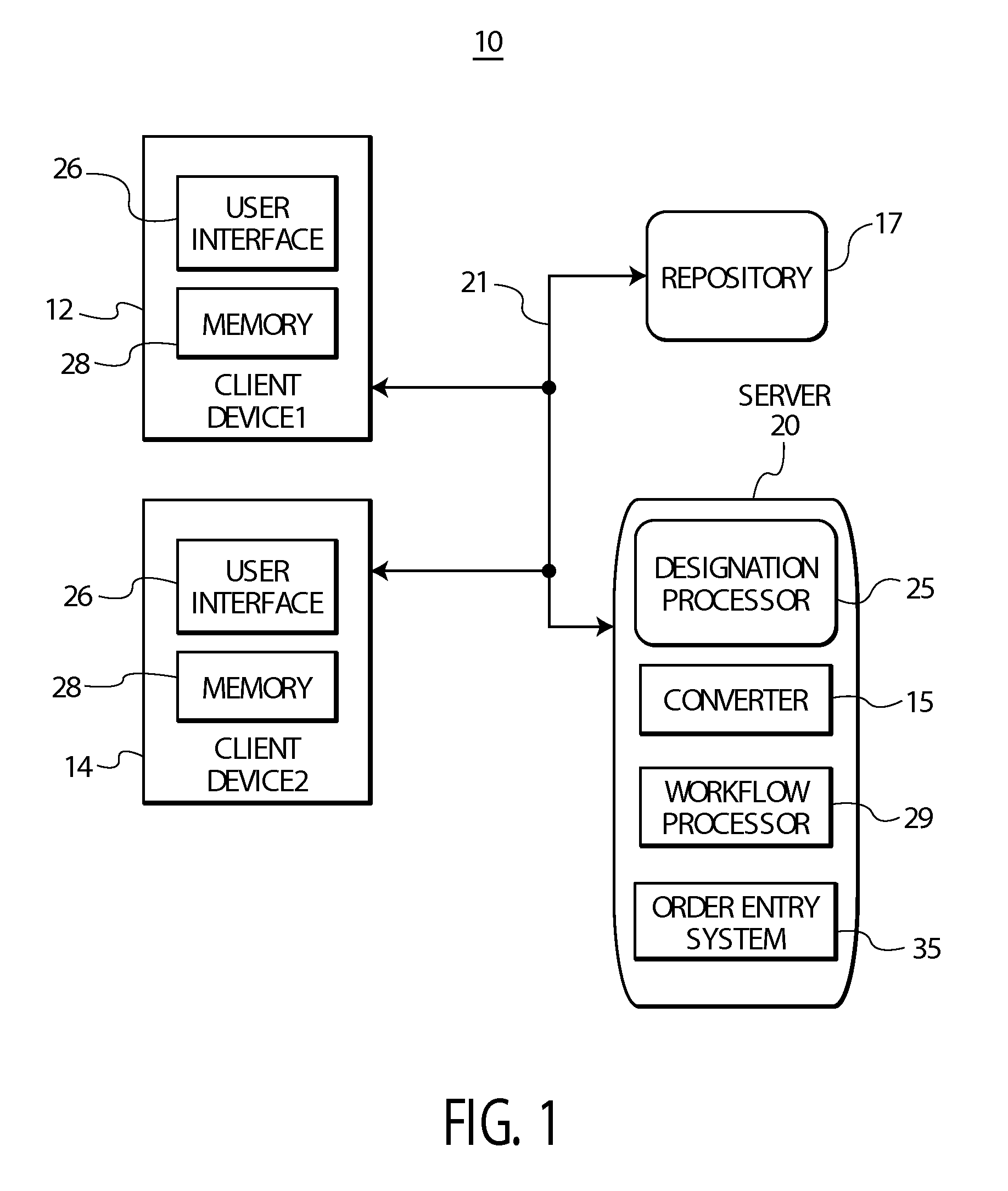
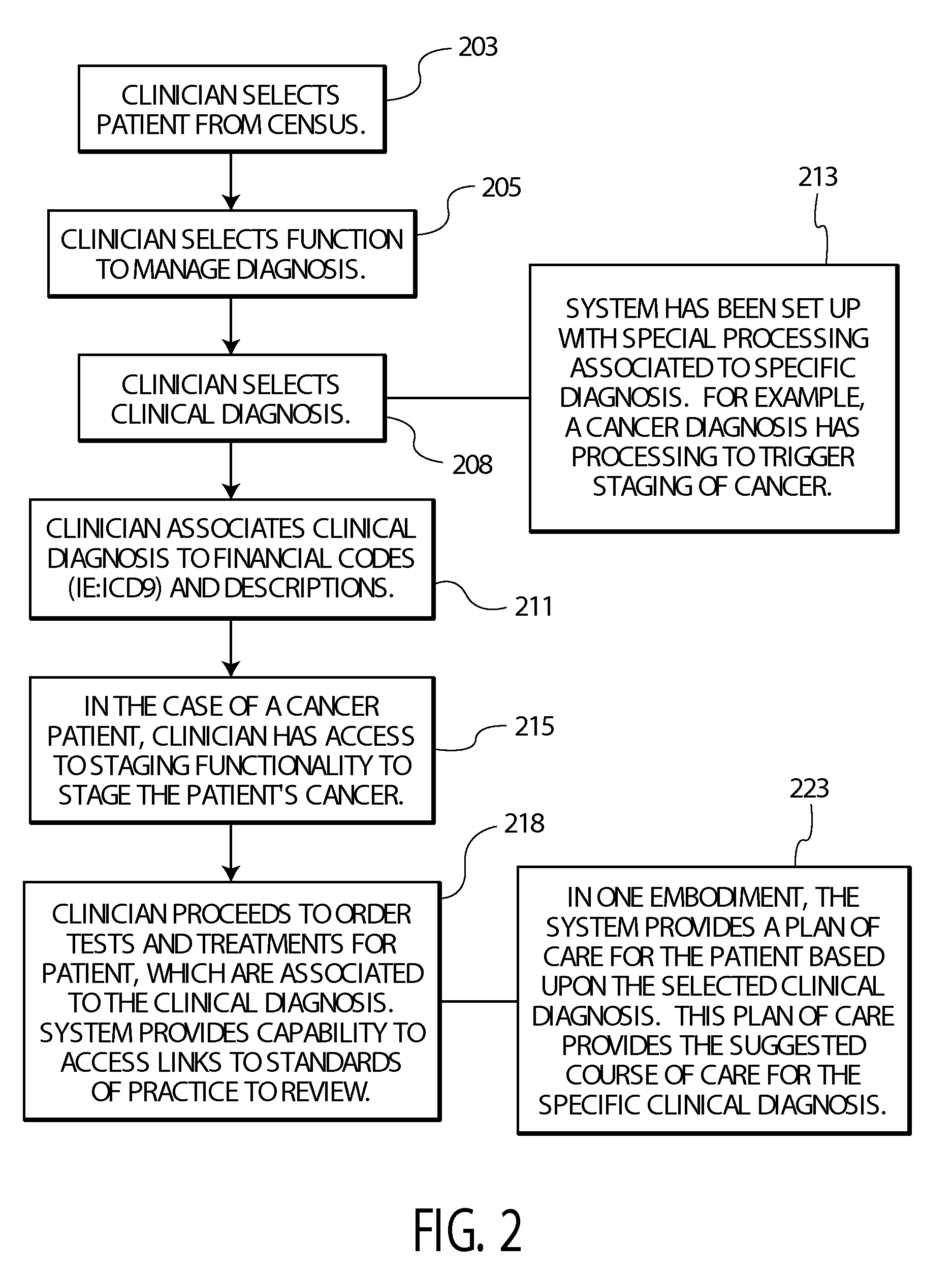
ActiveUS20090119128A1Data processing applicationsLocal control/monitoringEncounter identifierShort terms
A system provides an overview of patient medical condition by automatically converting a financial diagnosis description of a medical condition, into clinical terminology and associating a clinical diagnosis with a patient in a record that persists the association across multiple patient visits for a lifetime type medical condition. A system provides an overview of patient medical condition identifying both long term and short term medical conditions. The system includes a designation processor for designating an identified patient medical condition as being long term in response to user command. At least one repository stores information associating a designated long term patient medical condition with a patient and with a plurality of encounter identifiers identifying corresponding interactions of the patient with a healthcare provider organization and medical conditions associated with the encounters. A user interface initiates generation of data representing a composite display image window including data identifying a designated long term patient medical condition together with encounters and medical conditions associated with the encounters providing a user an overview of long term and short term medical conditions of a patient.
Owner:CERNER INNOVATION
Integrated medical software system with advanced patient scheduling
InactiveUS20110246226A1Cost-effective to implementCost-effective to maintainMedical report generationPatient personal data managementTime scheduleDisplay device
An integrated medical software system with a patient scheduling module for scheduling patient visits is disclosed. The module comprises a section where a user adds and removes clinical and administrative flags that are associated with patients and that are displayed as reminders when the patients schedule visits, said clinical flags alerting the user to a patient's medical issue that requires special attention and said administrative flags alerting the user to a patient's clerical issue that requires special attention, and a section where the user schedules equipment and a clinician for an appointment with a patient by performing the steps of selecting the patient at a first display point and the equipment at a second display point, said first and second display points being defined by different images that are viewable on a user interface display, and dragging the patient and the equipment to a third display point, said third display point being defined by an available time in the clinician's schedule that is viewable on the user interface display.
Owner:GREENWAY MEDICAL TECH
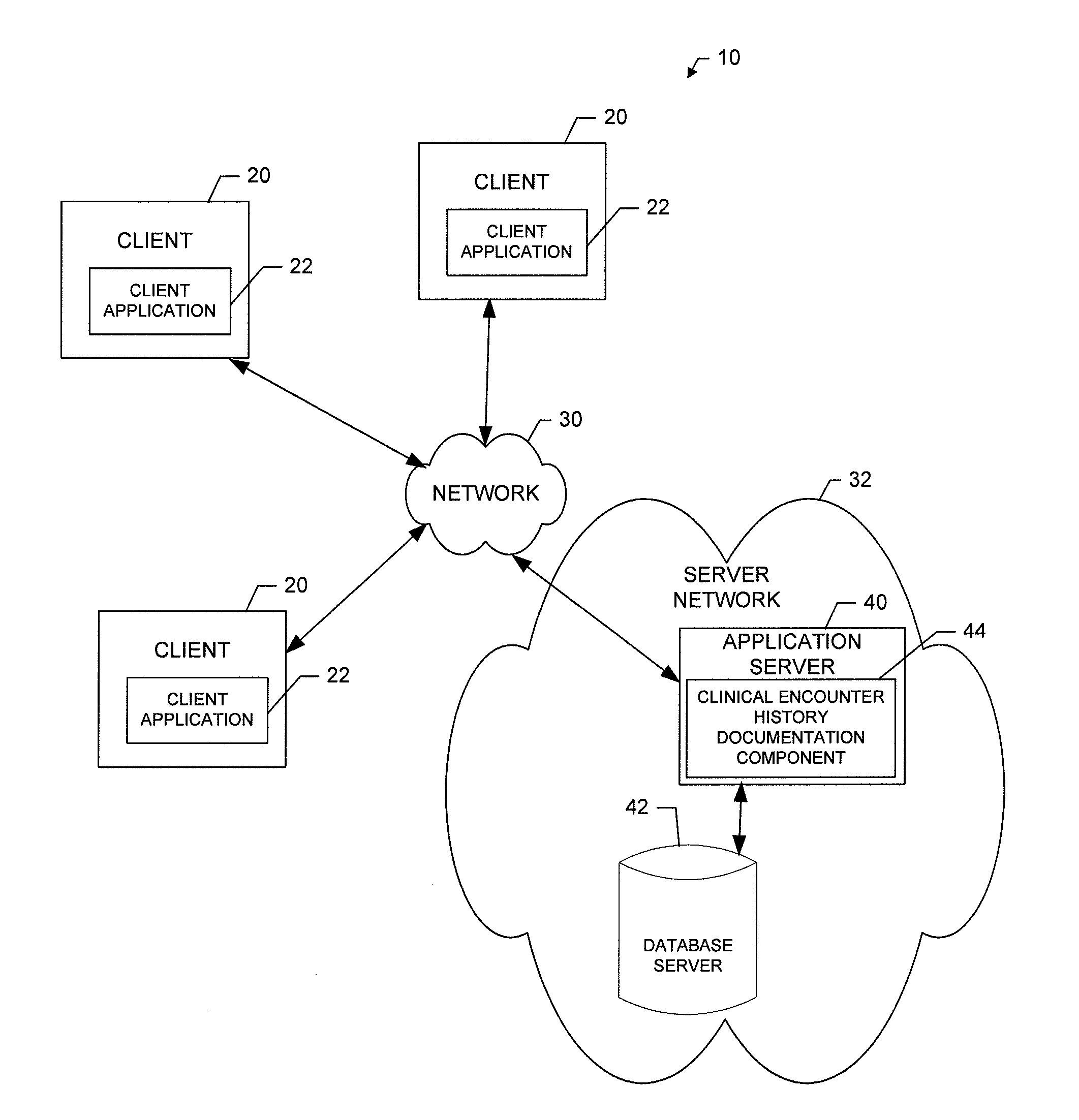
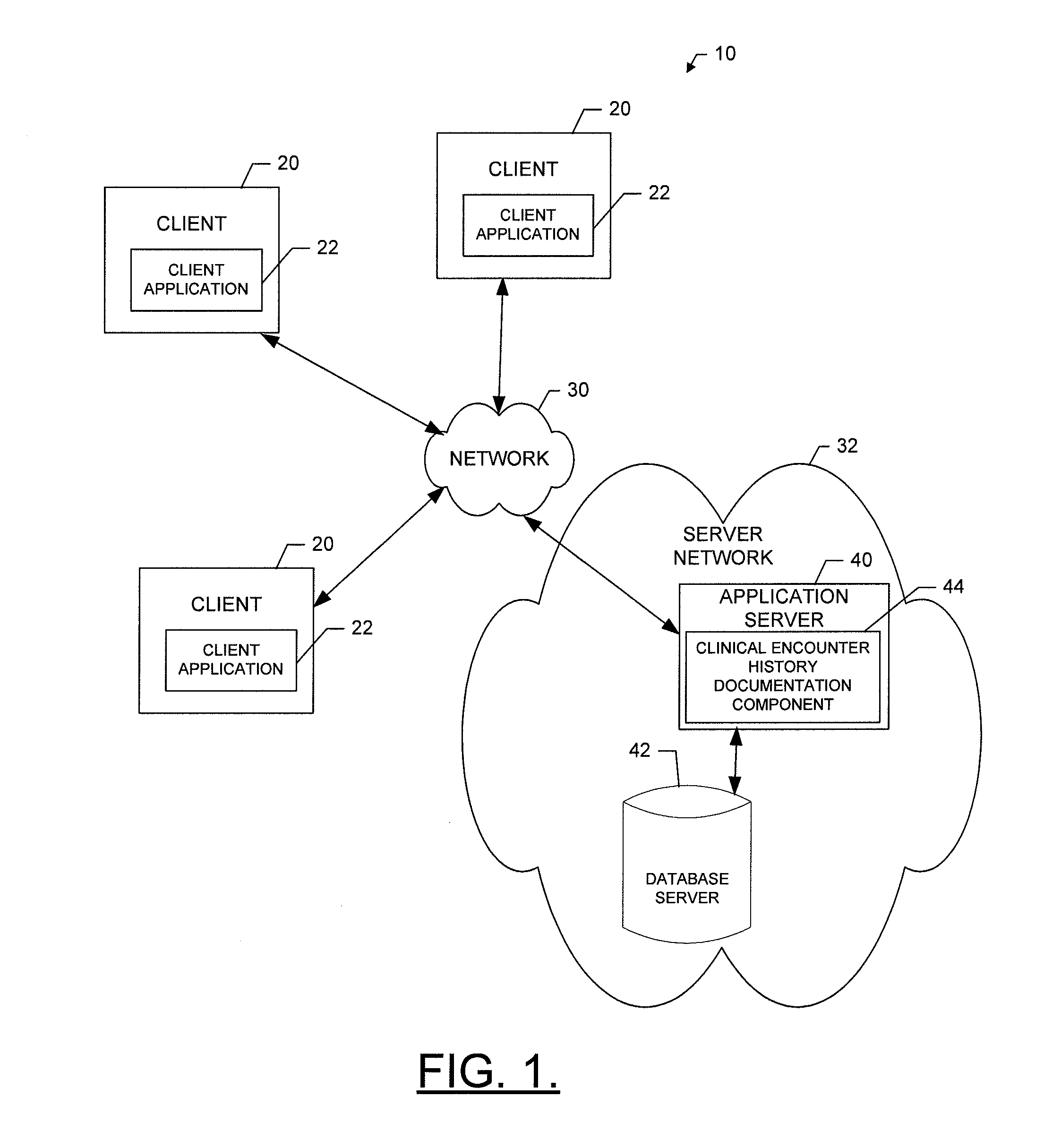
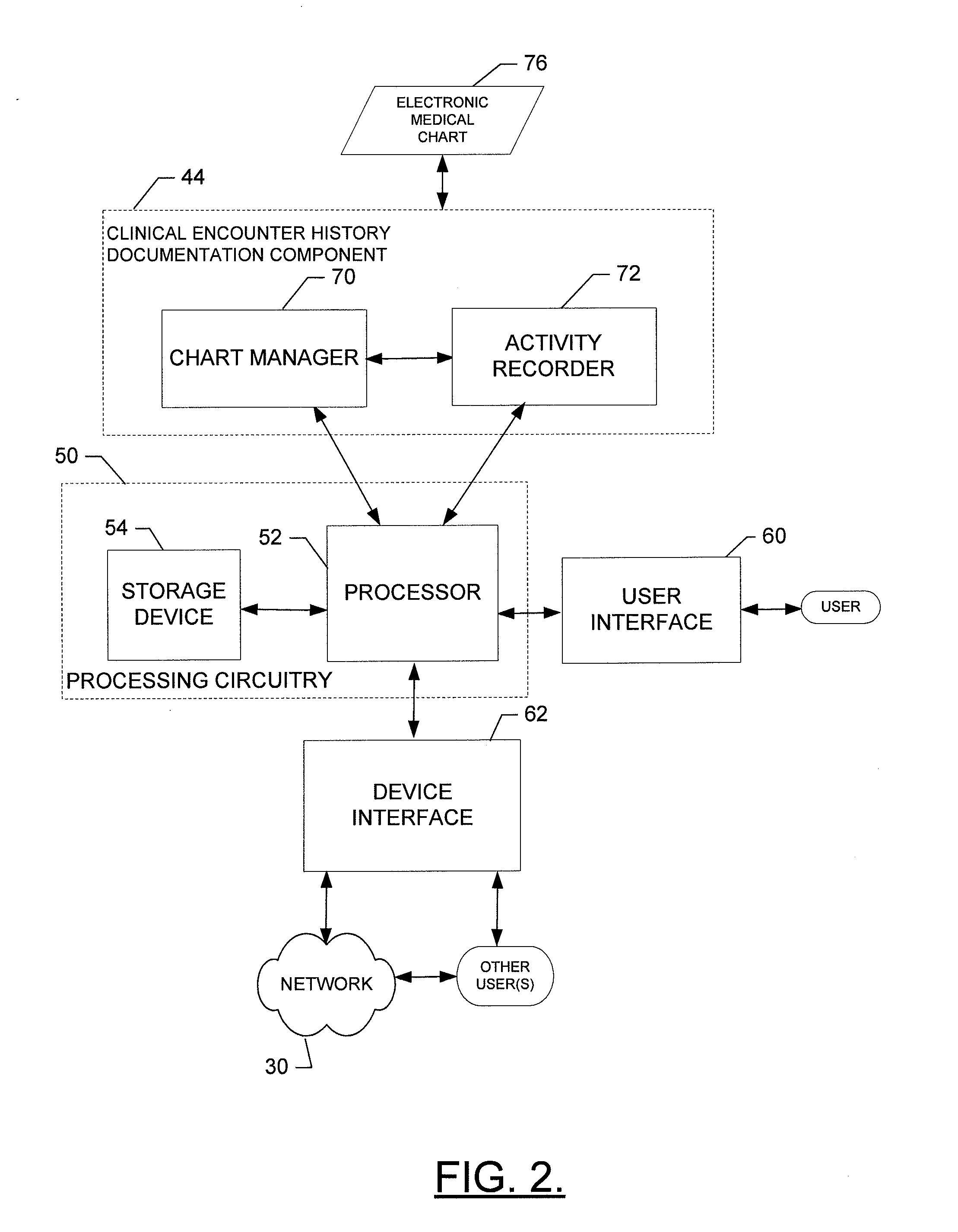
Method, apparatus and computer program product for providing documentation of a clinical encounter history
InactiveUS20120253841A1Efficiently navigateEfficient interpretationData processing applicationsHealthcare resources and facilitiesDocumentation procedureComputer science
A method for providing documentation of clinical encounter history may include providing for display of an electronic medical chart having a plurality of chart sections associated with respective different healthcare related topics associated with a patient, providing for display of an encounter history defining a set of activities performed in connection with a selected encounter where each activity of the set of activities is represented by a respective activity indicator and where the encounter history is dynamically updated to include an additional activity indicator for each respective activity that is undertaken in relation to a corresponding different chart section of a current patient visit, and enabling user selection of an activity indicator to link the user to a corresponding chart section related to the corresponding activity indicated by the activity indicator. A corresponding computer program product and apparatus are also provided.
Owner:MCKESSON FINANCIAL HLDG
System and method for making patient records follow a physician
ActiveUS20090299770A1Electric signal transmission systemsDigital data processing detailsPhysician patientEXAMINATION ROOM
A computer-based system for providing physicians automatic, secure access to patient records at the time a patient visits and consults a physician. The system can include one or more computing devices configured to process and display data. The system can also include one or more emitting devices carried by physicians for identifying each particular physician and one or more scanning devices configured to detect and communicatively link to the one or more emitting devices based on the proximity of the physician to an examination room. Additionally, the system can include a manager module communicatively linked to the one or more scanning devices and configured to manage the signal data from the one or more scanning devices. Furthermore, the system can also include one or more servers communicatively linked with the one or more computing devices, one or more scanning devices, and manager module, the one or more servers configured to authenticate and register a particular patient upon a visit to a hospital or office, synchronize the particular physician identifier, patient identifier, personal wellness electronic record (PWER), and examination room identifier based upon physician's proximity to an examination room, initiate a physician-patient session at the at least one computing device at the examination room, transfer the PWER to the at least one computing device at the examination room, and automatically terminate the physician-patient session and receive the PWER based upon the physician leaving the proximity of the examination room.
Owner:THE QUANTUM GROUP +1
Personal respiratory protection system
A respiratory protection system which is worn by a passenger during a flight in air plane, by a patient visiting hospital or doctors office, a nurse working with a patient during hospital stay, or the like. The system includes a relatively light weight, substantially rigid, headgear structure, containing an air disinfection chamber, a fan means, a filter means, an ultraviolet air disinfection means, an air blanket origination means and an energizing means.
Owner:GLAZMAN MARK
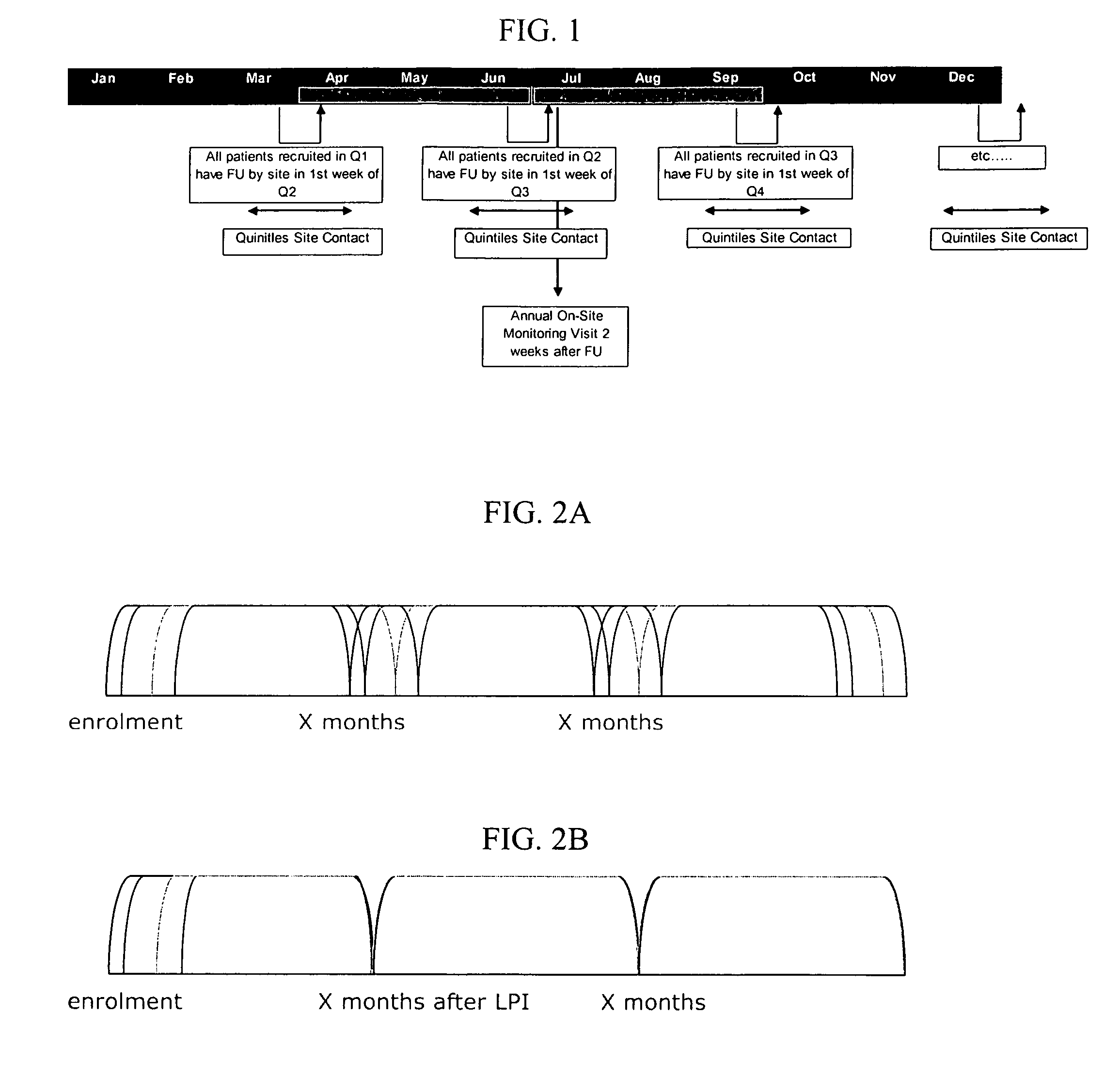
Systems and methods for scheduling and sequencing sessions or appointments
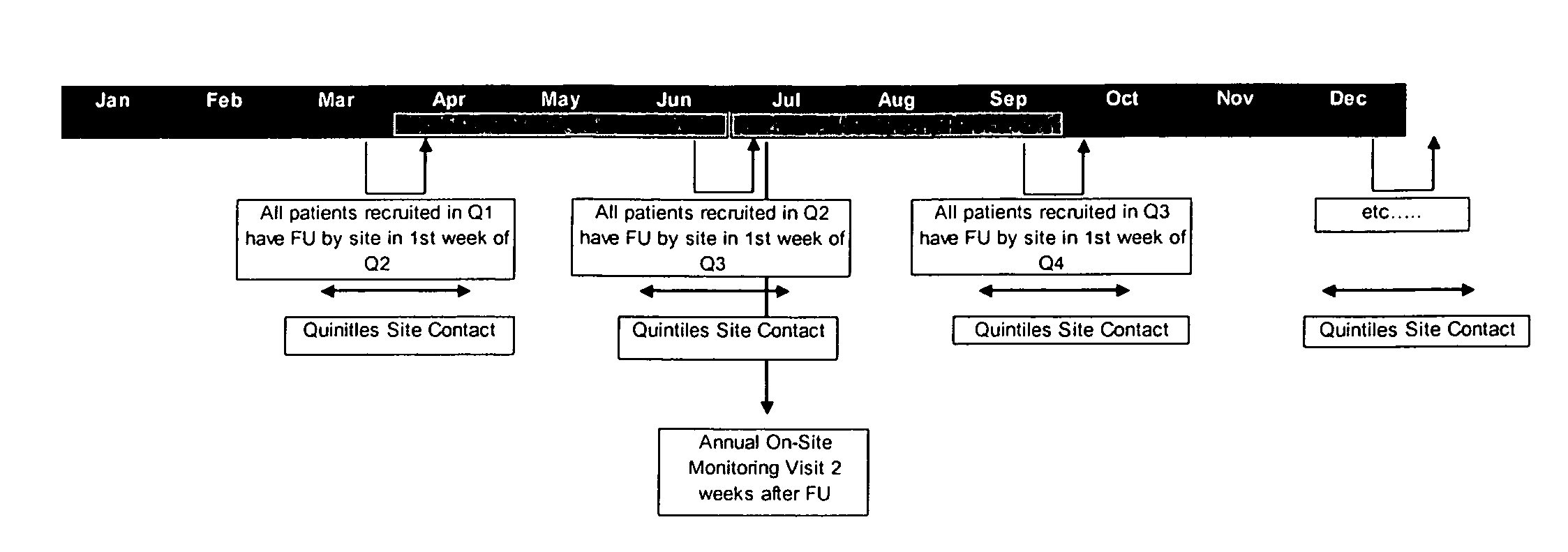
Embodiments of the present invention provide systems and methods for scheduling patients in an organized and uniform way using a batch follow-up system. Such batch follow-up methods help a research site conducting research to prepare one or more of study supplies, patient charts, study goals, and data collecting requirements in preparation for the batch follow-up visits. Embodiments of the invention also relate to systems and methods for communicating initial and follow-up visit dates to patients. In certain instances, various communications (e.g., between the patients and the study site or between the study site and the study sponsor) may be electronic communications. Methods of scheduling and sequencing patient visits may be conducted over a network wherein reminders about follow-up visits are electronically generated.
Owner:IQVIA INC
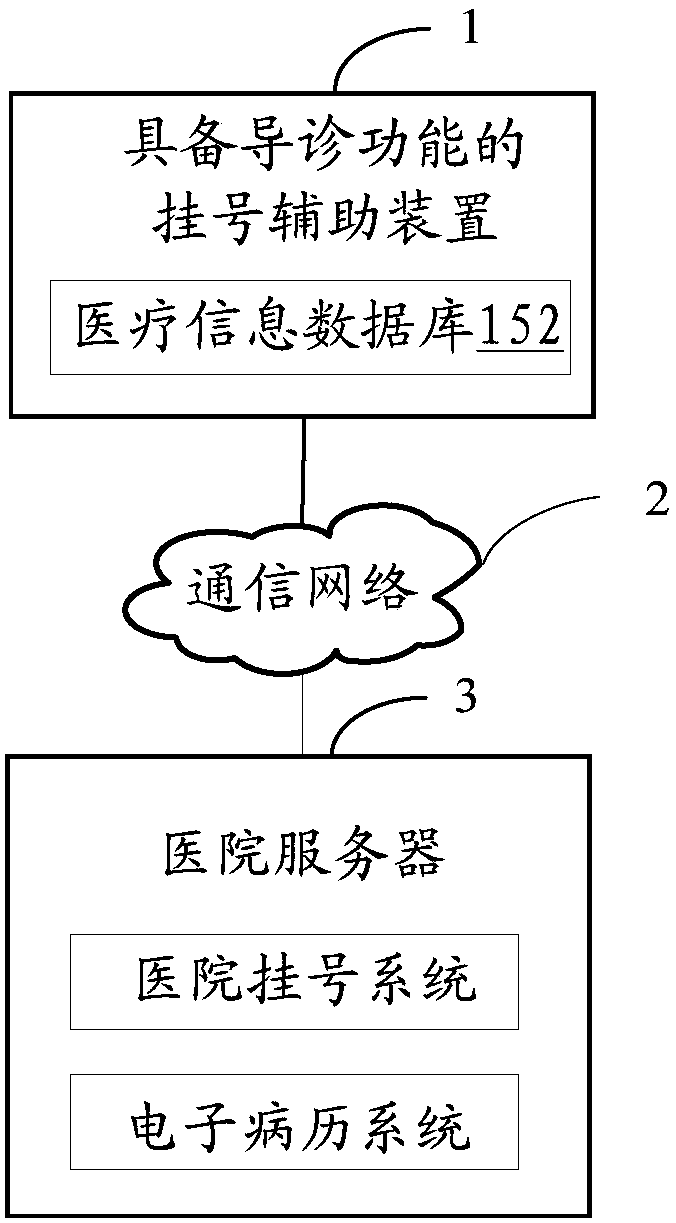
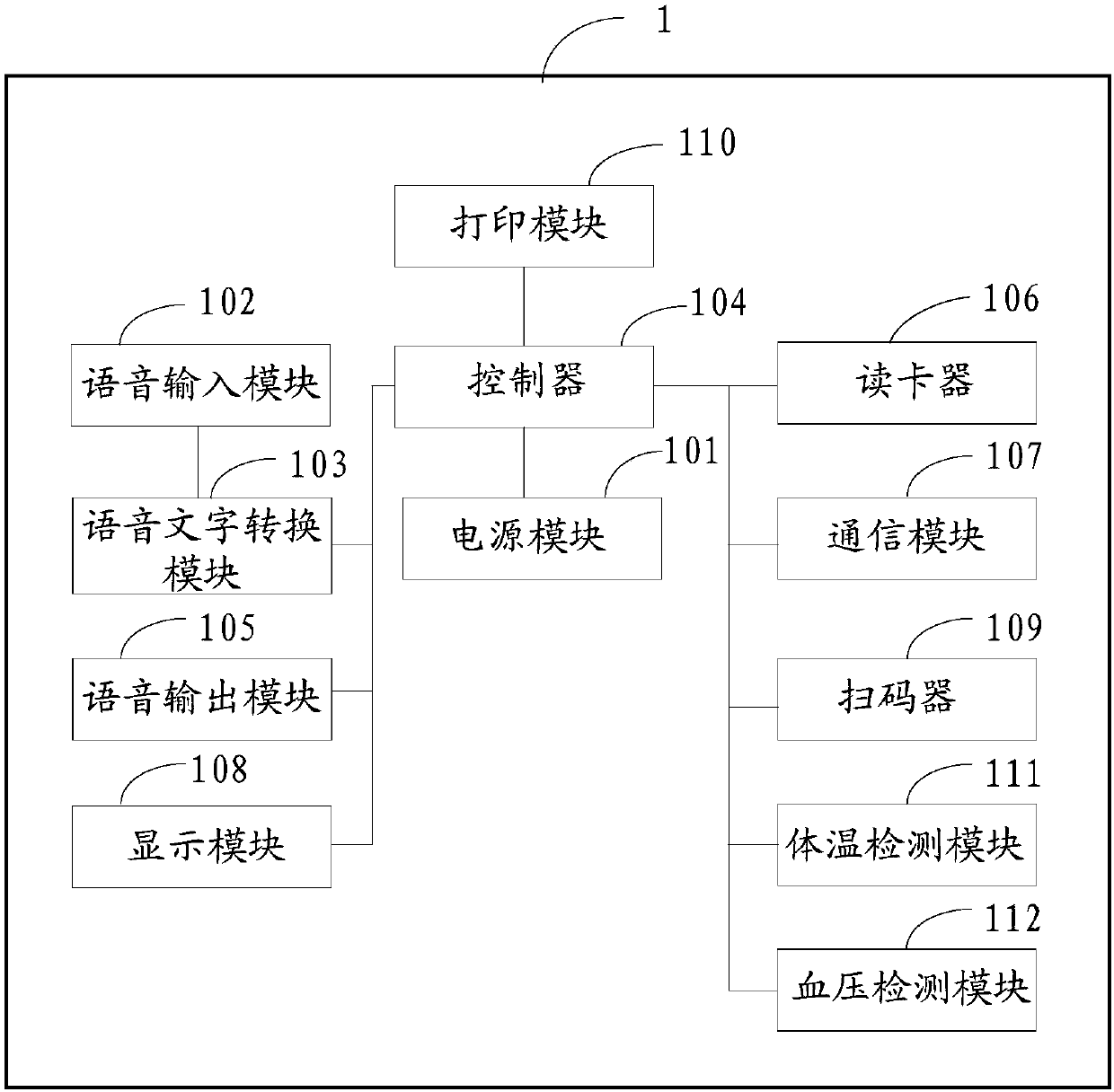
Auxiliary registration device with hospital guiding function
InactiveCN107818625AImprove registrationImprove the efficiency of medical treatmentData processing applicationsMedical automated diagnosisPatient VisitSpeech sound
The invention discloses an auxiliary registration device with a hospital guiding function. The auxiliary registration device with the hospital guiding function is characterized in that voice messagesof patients are collected, and the collected voice messages of the patients are converted into text messages of the patients; key words are extracted from the text messages of the patients, and the extracted key words are compared with a medical information database; when the key words are matched with the medical information database, patient symptom key words contained in the key words are determined; according to the patient symptom key words, patient visiting department information is inquired from the medical information database, identity information of the patients is collected, and theidentity information of the patients, the patient visiting department information and patient registration requests are sent to a hospital server; furthermore, the collected patient body temperaturevalues, patient blood pressure and patient text information are sent to the hospital server. The auxiliary registration device with the hospital guiding function disclosed by the invention can improveregistration and doctor visiting efficiencies and improve user experience.
Owner:E TECHNO INFORMATION TECH +1
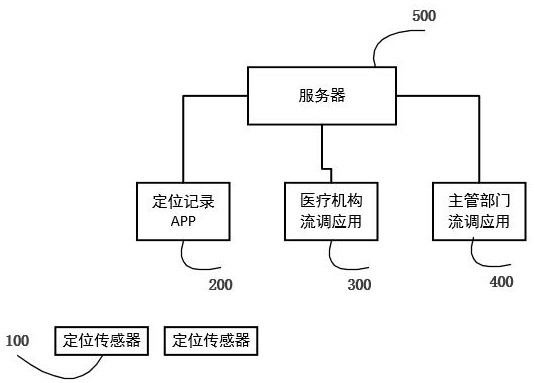
Epidemic disease investigation method and system based on public place positioning sensor
ActiveCN111785393ALow costPerformance requirements are easy to meetParticular environment based servicesEpidemiological alert systemsPublic placeEngineering
The invention discloses an epidemic disease investigation method and system based on a public place positioning sensor. The system comprises a positioning sensor, a positioning record App, a server, ahospital traffic scheduling application and a competent department traffic scheduling application. The positioning sensor is mounted in a public place; the positioning record App is installed in a personal mobile phone, and after the broadcast sent by the positioning sensor is scanned and the verification information is checked to be valid, the positioning record information is locally stored inthe mobile phone. When a patient visits a medical institution, a hospital can export positioning record information from the personal mobile phone of the patient according to epidemic prevention and control regulations, and early unknown infectious diseases can be discovered through a patient position correlation analysis report; epidemic investigation competent departments can use positioning record information exported from personal mobile phones of epidemic patients and issue cooperation investigation electronic notifications, and people whose local positioning information of the mobile phones is within the range of the electronic notifications can receive the notifications.
Owner:蒋芳
Systems and methods for capturing data, creating billable information and outputting billable information
ActiveUS20160239617A1Well formedDigital data information retrievalPatient personal data managementExternal dataUnstructured data
Methods, systems, and computer-readable media are provided for generating and outputting medical billing data. Data is captured by pervasive devices before, during, and after a patient encounter with a healthcare provider. Any unstructured data is transformed into usable structured data, and determinations, such as a service provided by the healthcare provider, are made based on that data as well as other stored data. Based on those determinations, billing data for billing claims related to the patient visit is generated and outputted to a payer of the claims. The billing data may include a medical code that is predicted by a billing model. Billing models may be created from data captured by the pervasive devices from patient visits and data in external data stores, such as electronic health records.
Owner:CERNER INNOVATION
System and method for supervising patient
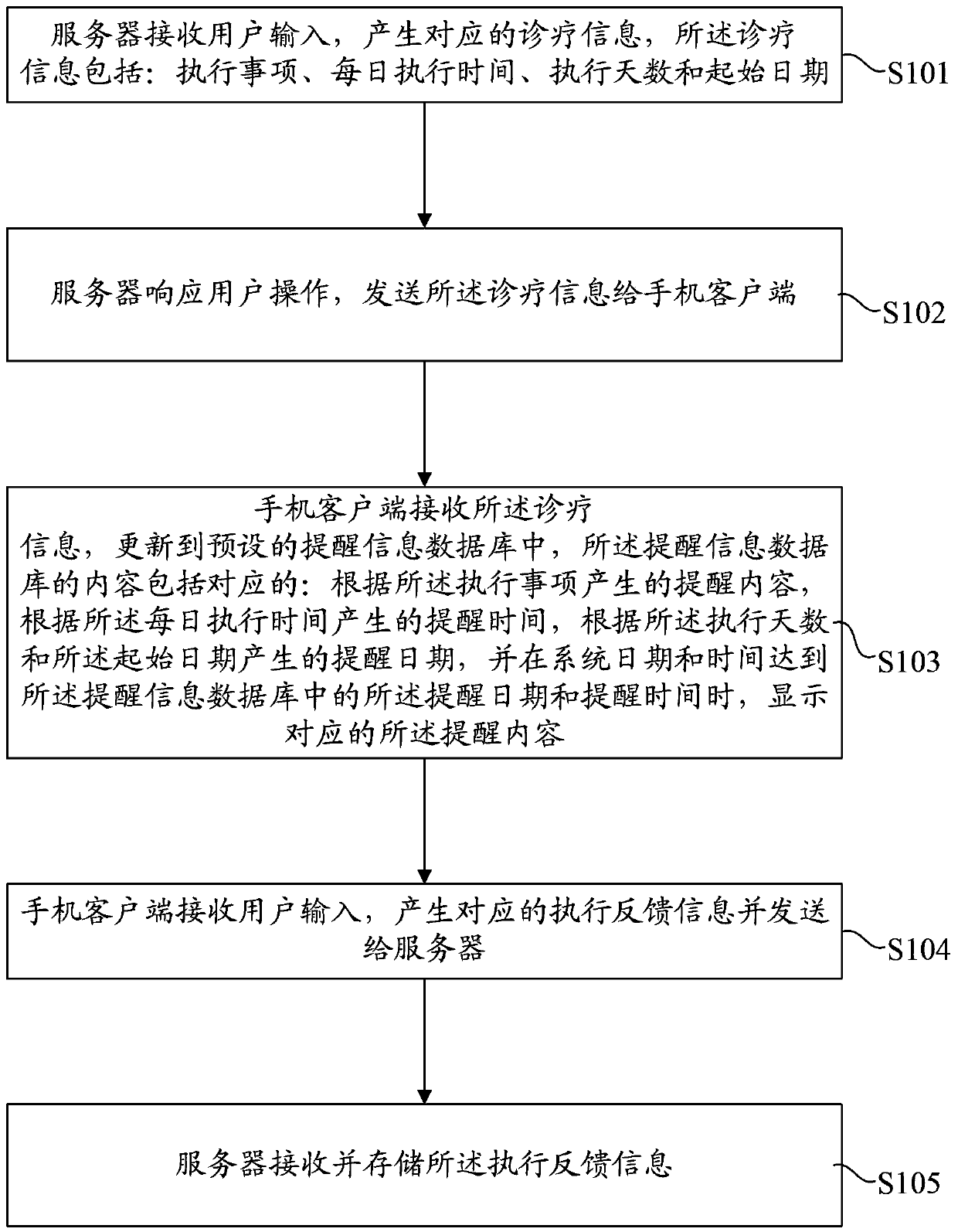
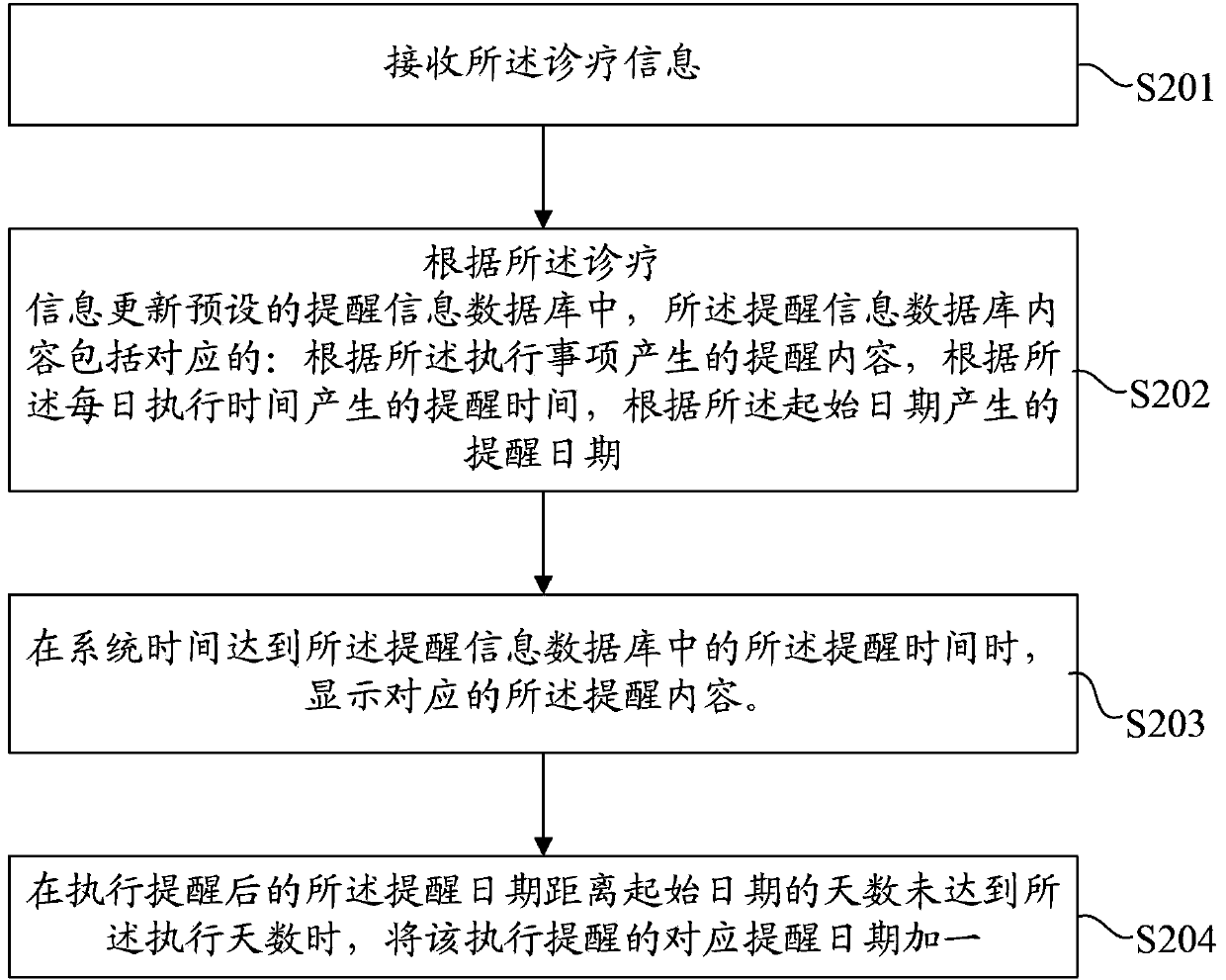
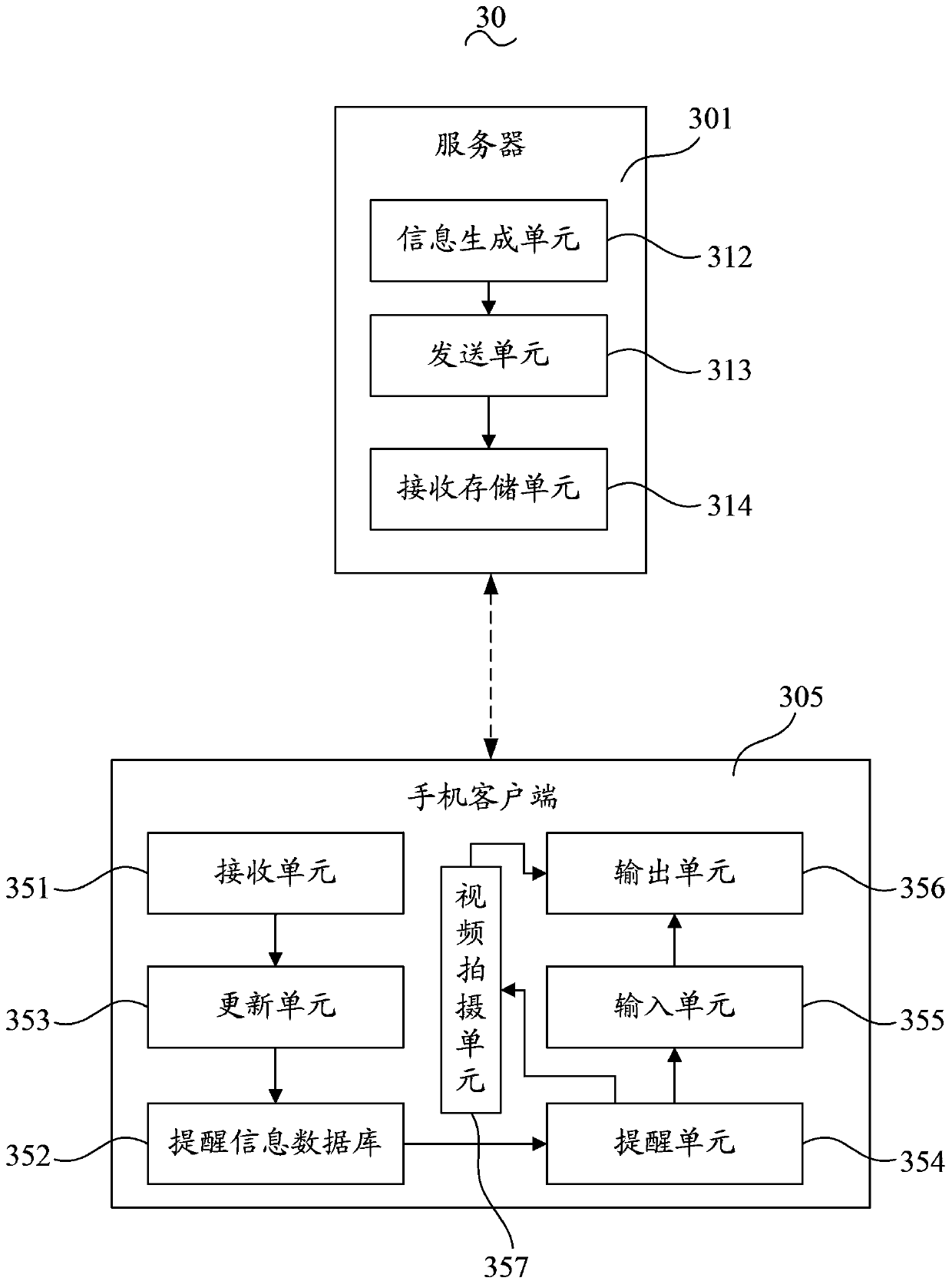
InactiveCN103426134APrivacy protectionImprove treatment efficiencyData processing applicationsUser inputClient-side
The invention discloses a method for supervising a patient. The method includes that a server receives input information of a user and generates treatment information, and the treatment information includes execution matters, daily execution moments, the number of execution days and a starting date; the server transmits the treatment information to a mobile phone client; the mobile phone client receives the treatment information, the treatment information is updated into a preset prompting information database, contents of the prompting information database include prompting contents, prompting moments and prompting dates which correspond to one another, and the corresponding prompting contents are displayed when system dates and moments are identical to the prompting dates and the prompting moments in the prompting information database; the mobile phone client generates corresponding execution feedback information and transmits the corresponding execution feedback information to the server. The invention further provides a corresponding system for supervising the patient. The method and the system for supervising the patient have the advantages that the traditional form that patients visit hospitals or medication branches for treatment prompting, supervision and feedback is replaced by a mobile communication network, so that the treatment efficiency is effectively improved, and the privacy of the patient is effectively protected.
Owner:HUIZHOU CITY TUBERCULOSIS INST
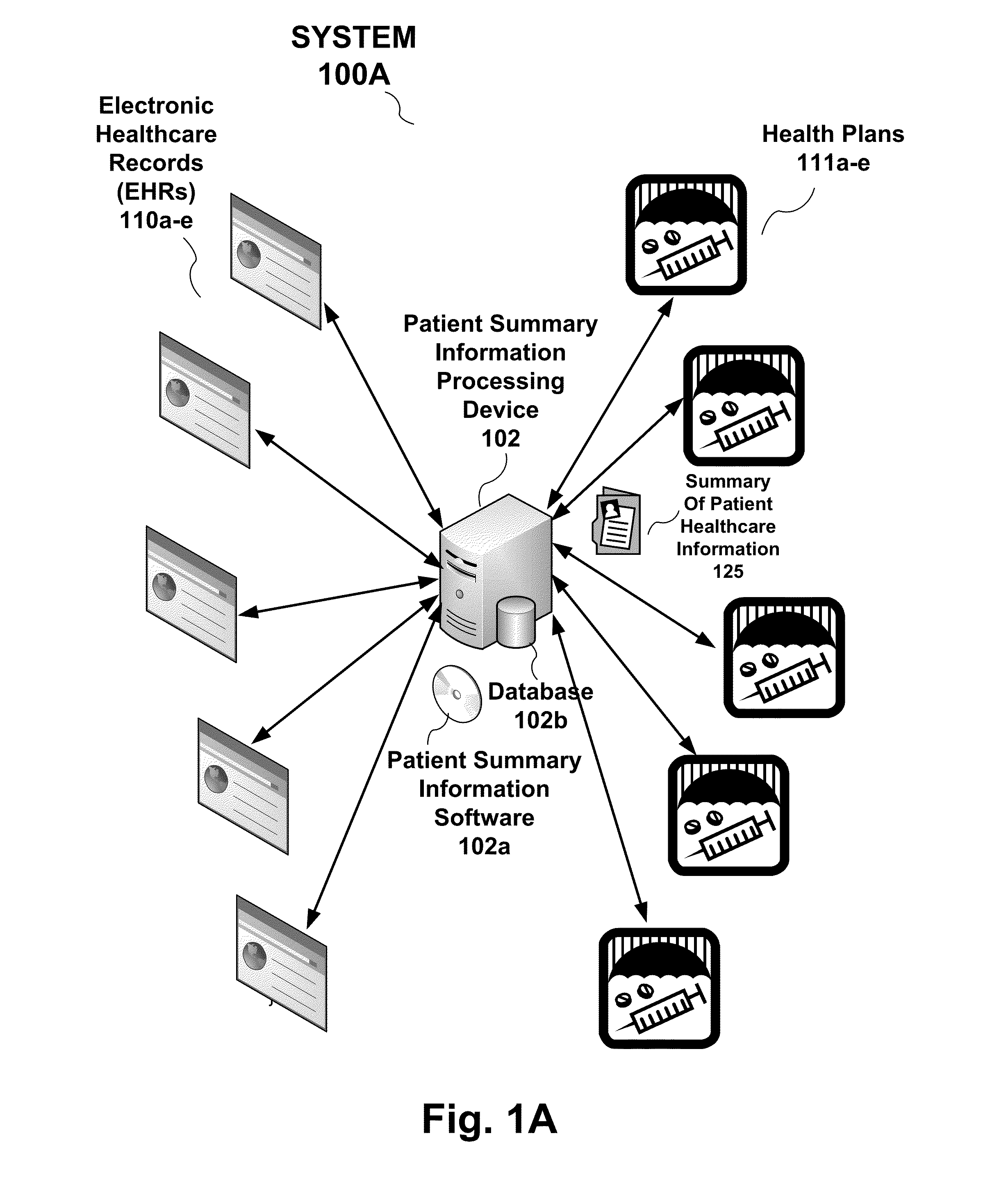
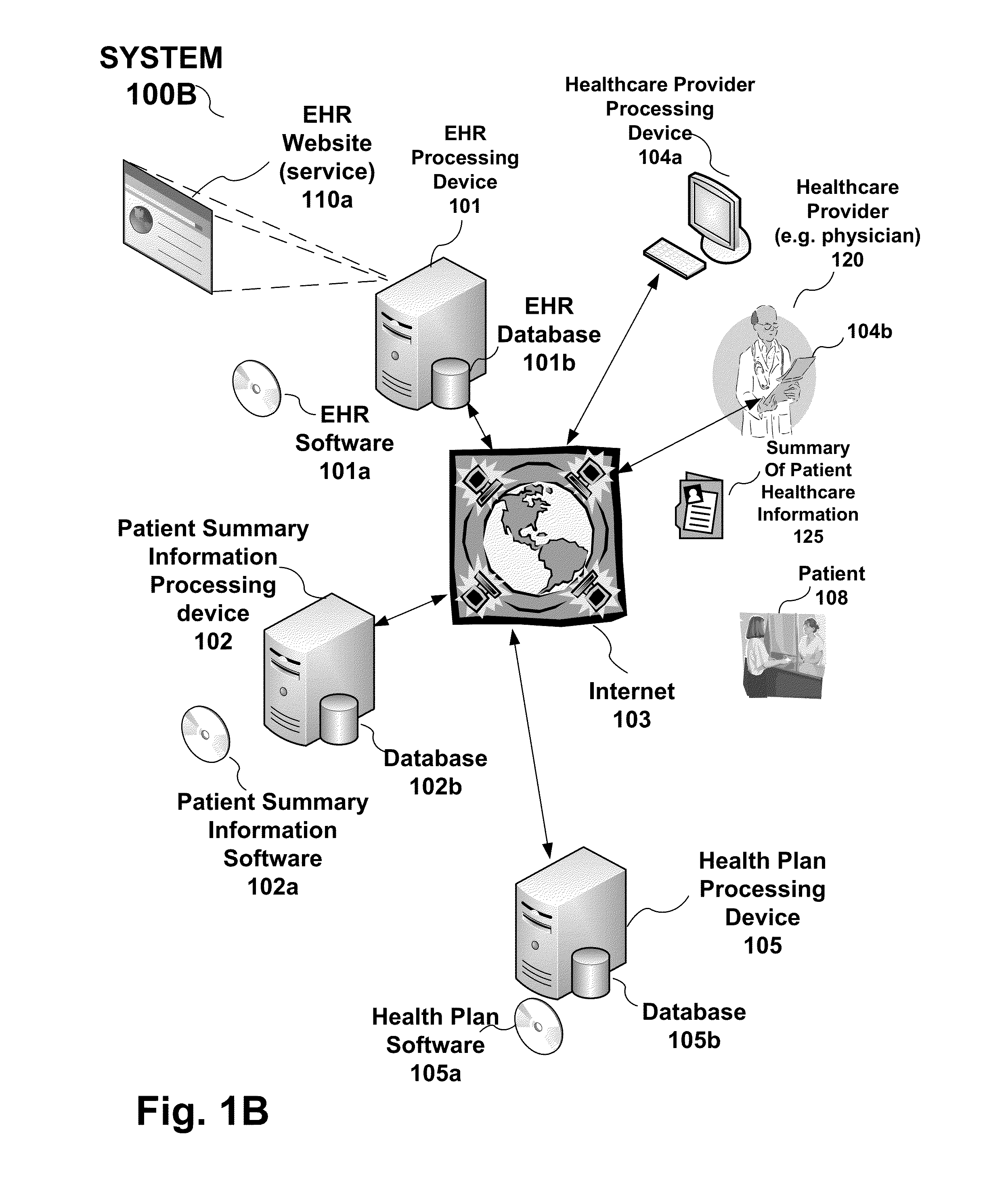
System, processing device and method to provide a summary of patient healthcare information to an electronic health record from a health plan provider
Owner:PDR NETWORK
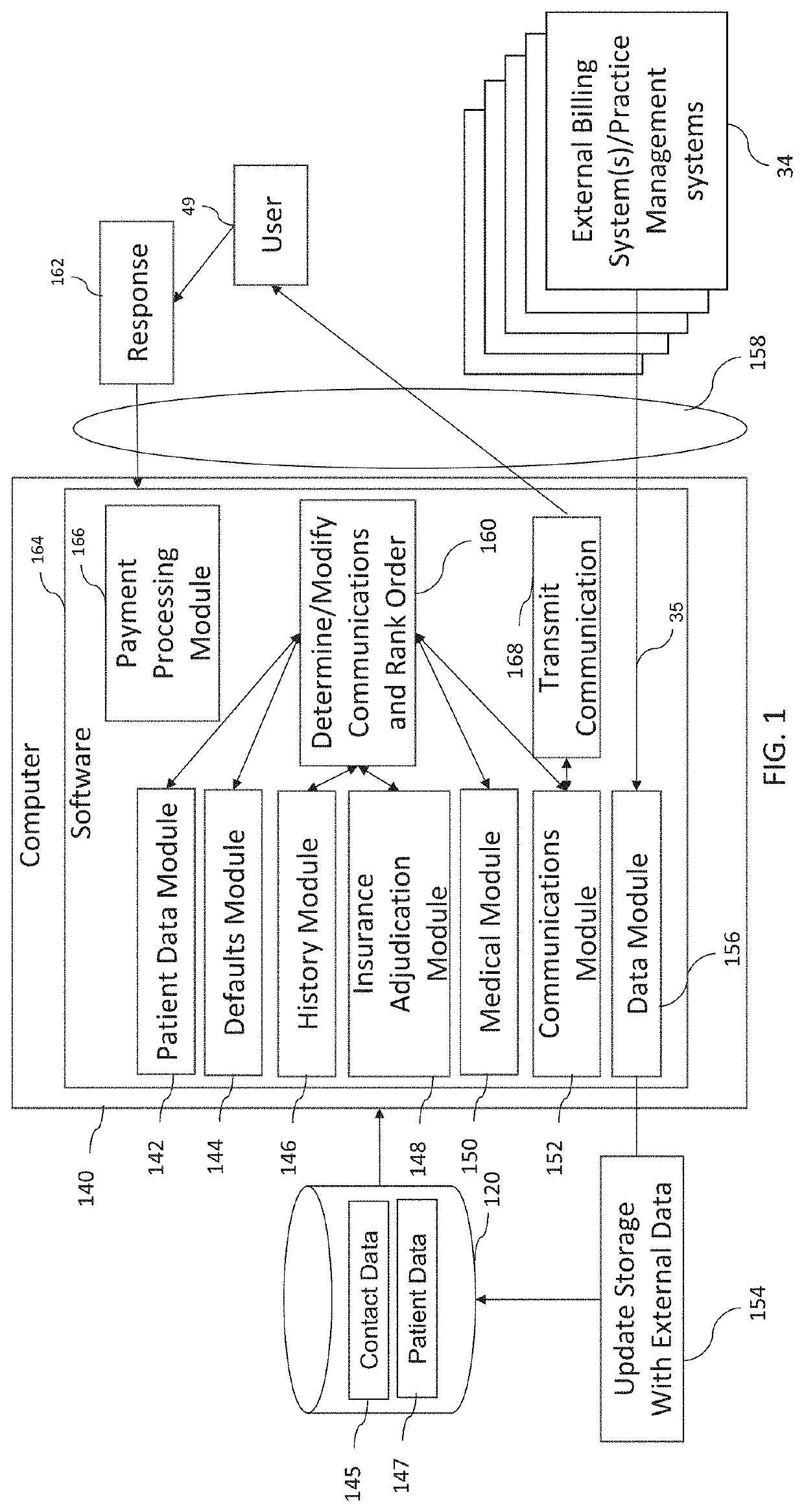
Intelligent Patient Billing Communication Platform For Health Services
ActiveUS20200019946A1Improve administrative efficiencyImprove the accuracy of patient billingPayment architecturePatient-specific dataEngineeringContext data
A system for generating customized patient billing communications includes software executing on a server which receives patient billing data indicative of a patient visit and further indicative of a balance. The software accesses a storage to determine patient visit context data indicative of one or more visit codes associated with the billing data. The software generates a communication for a user based on the patient visit context data with message content altered from a standard message based on the one or more visit codes, the communication including a bill for the balance and being a first communication to the user with the bill.
Owner:INBOX HEALTH CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com