The preparation method of omeprazole intermediate
A volume ratio, dimethyl technology, applied in the direction of organic chemistry, can solve the problems of unfavorable industrial scale production, affecting production efficiency and product yield, low purity, etc., to reduce the impurity content, reduce the difficulty of purification, and simplify the purification process Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Embodiment 1 (0.03% silver nitrate)
[0064]
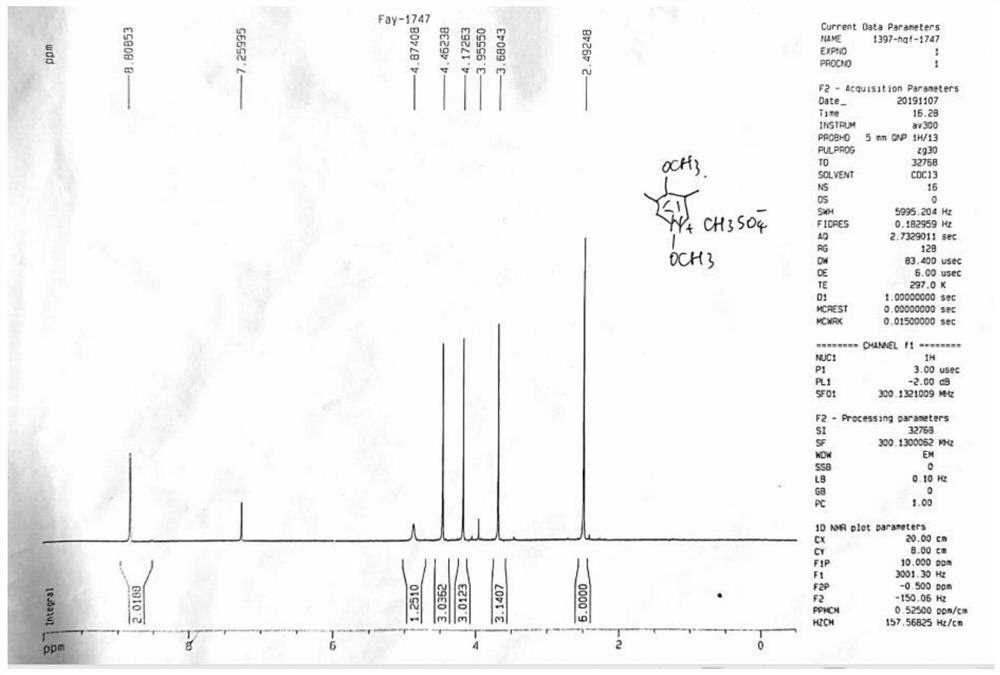
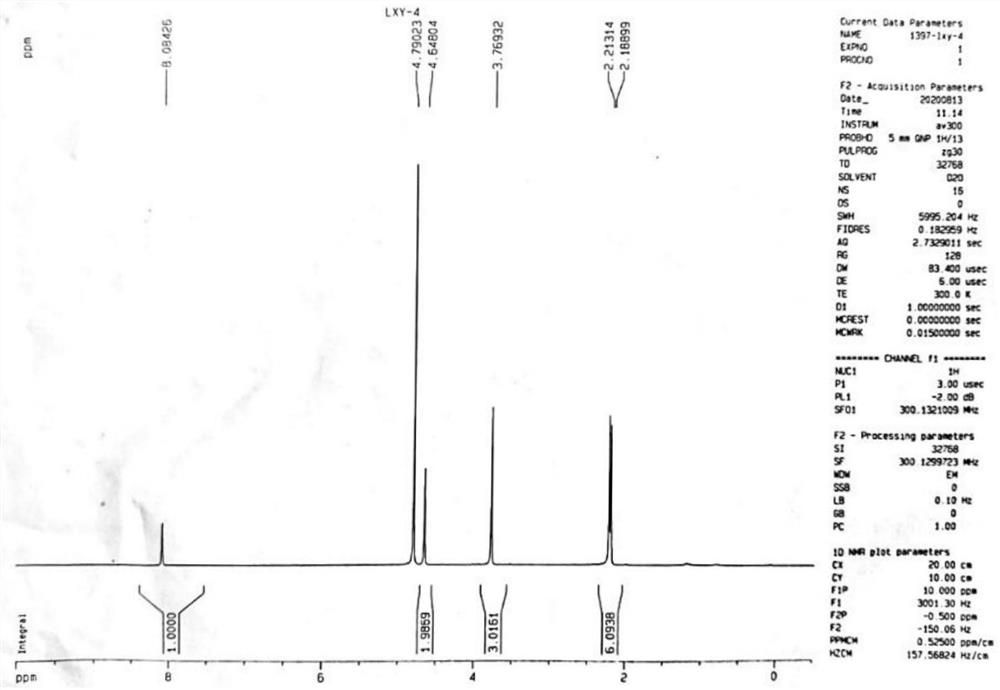
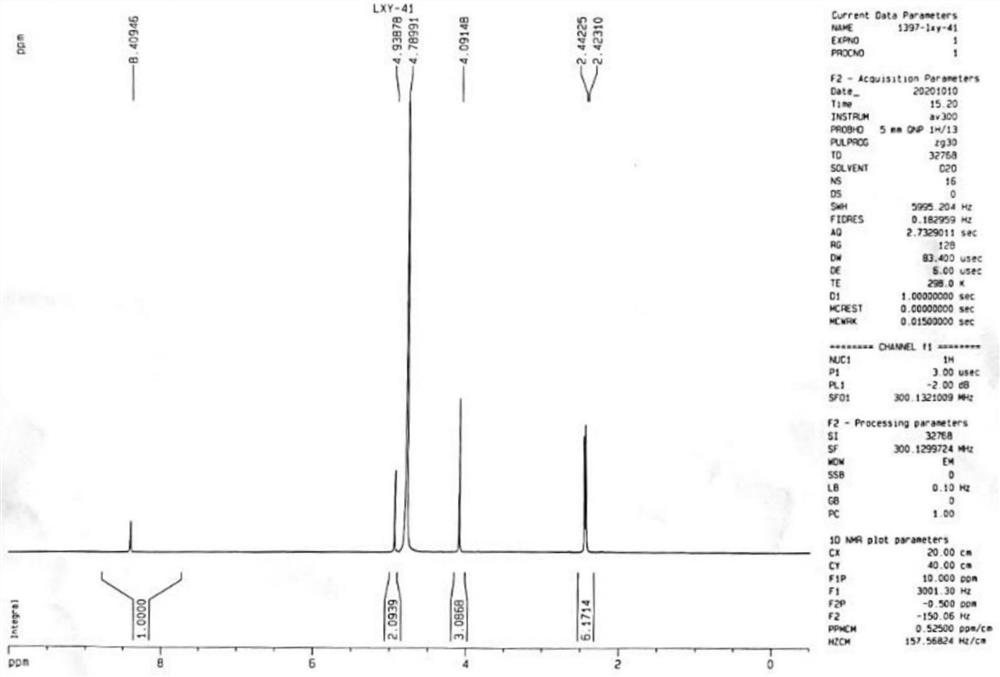
[0065] Step 1: Take compound II-1 (4.9g, 17.56mmol), add 20mL water and 30mL methanol, stir and dissolve evenly, add silver nitrate (0.9mg, 0.0053mmol, 0.03%), and heat up to reflux. Ammonium persulfate solution (4.01g, 17.57mmol, ammonium persulfate dissolved in 16mL of water) was added dropwise to the reaction system for a total of 2 hours. After the drop was completed, the reaction was continued for 3h under reflux conditions, the reaction was stopped, and 30 ml of methanol was added , filtered, the filtrate was concentrated under reduced pressure to remove methanol, the residue was adjusted to pH 10 with 10mol / L NaOH solution in an ice-water bath, the aqueous phase was extracted with dichloromethane, the organic phases were combined, dried with anhydrous sodium sulfate, and concentrated to obtain compound III (2 -hydroxymethyl-3,5-dimethyl-4-methoxypyridine) to obtain 2.90 g of brown solid, yield: 98.8%.
[0066] St...
Embodiment 2
[0067] Embodiment 2 (0.005% silver nitrate)
[0068]
[0069] Step 1: Take compound II-1 (4.9g, 17.56mmol), add 20mL water and 30mL methanol, stir and dissolve evenly, add silver nitrate (0.15mg, 0.005%), and heat up to reflux. Ammonium persulfate solution (4.01g, 17.57mmol, ammonium persulfate dissolved in 16mL of water) was added dropwise to the reaction system for a total of 2 hours. After the drop was completed, the reaction was continued for 3h under reflux conditions, the reaction was stopped, and 30 ml of methanol was added , filtered, the filtrate was concentrated under reduced pressure to remove methanol, the residue was adjusted to pH 10 with 10mol / L NaOH solution in an ice-water bath, the aqueous phase was extracted with dichloromethane, the organic phases were combined, dried with anhydrous sodium sulfate, and concentrated to obtain compound III (2 -hydroxymethyl-3,5-dimethyl-4-methoxypyridine) to obtain 2.85 g of brown solid, yield: 97.2%.
[0070] Step 2: Add...
Embodiment 3
[0071] Example 3 (methanol / water volume ratio is 1:1)
[0072]
[0073] Step 1: Take compound II-1 (4.9g, 17.56mmol), add 14mL water and 30mL methanol, stir and dissolve evenly, add silver nitrate (0.9mg, 0.0053mmol, 0.03%), and heat up to reflux. Ammonium persulfate solution (4.01g, 17.57mmol, ammonium persulfate dissolved in 16mL of water) was added dropwise to the reaction system for a total of 2 hours. After the drop was completed, the reaction was continued for 3h under reflux conditions, the reaction was stopped, and 30 ml of methanol was added , filtered, the filtrate was concentrated under reduced pressure to remove methanol, the residue was adjusted to pH 10 with 10mol / L NaOH solution in an ice-water bath, the aqueous phase was extracted with dichloromethane, the organic phases were combined, dried with anhydrous sodium sulfate, and concentrated to obtain compound III (2 -hydroxymethyl-3,5-dimethyl-4-methoxypyridine) to obtain 2.84 g of brown solid, yield: 96.8.0%....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com