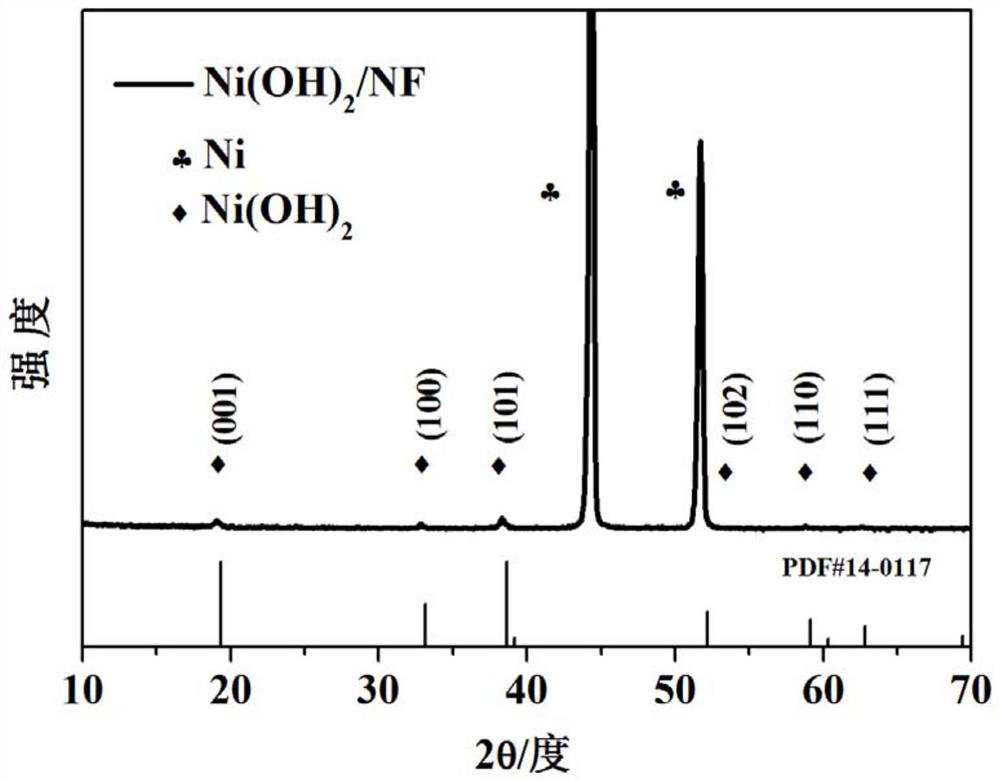
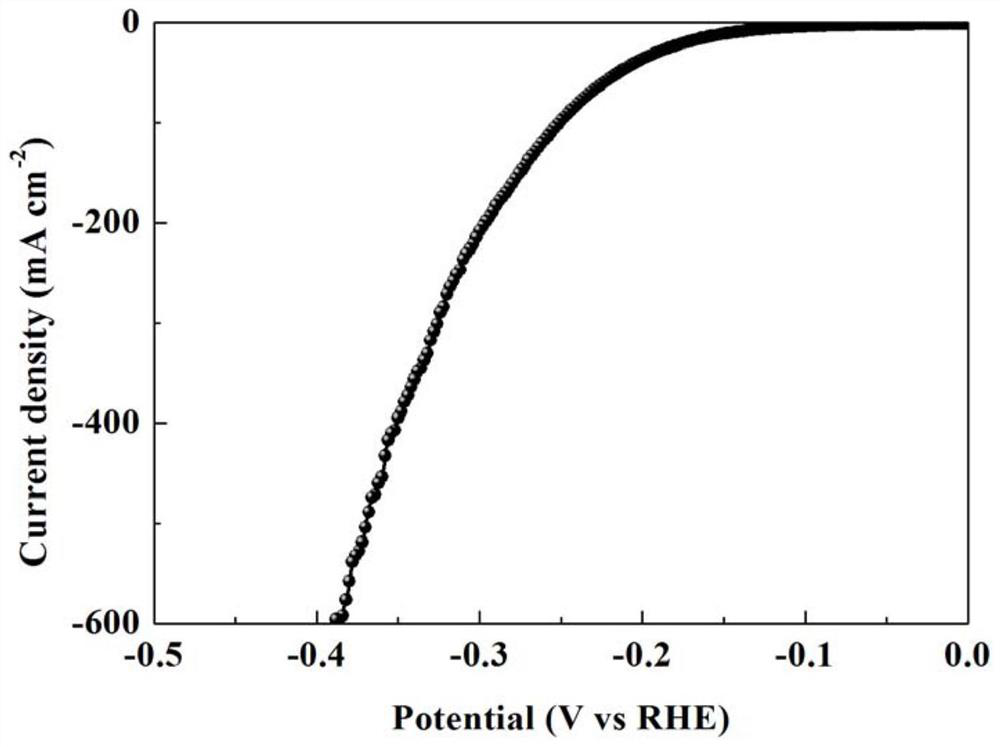
A chromium-vanadium co-doped nickel-based hydroxide self-supporting electrode for total water splitting and its preparation method
A self-supporting electrode and hydroxide technology, applied in electrodes, electrolysis components, electrolysis processes, etc., can solve the problems of high price and low reserves, and achieve the effect of increasing surface area, enhancing catalytic activity, and enhancing mechanical stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] The preparation method of the preparation of a chromium-doped nickel-based hydroxide self-supporting electrode of the present invention, and the specific steps include:
[0026] Step 1: Pretreatment of foam nickel. The foamed nickel was cut into 1 cm × 5 cm block, and it washed it in acetone solution for 10 min, then poured into a well-equipped 3 mol / L hydrochloric acid solution for 5 min, and finally washed with anhydrous ethanol and ultrapure water. 3 times, then dry at 25 ° C for 12 hours;
[0027] Step 2: 170 mg of hexahydrate nickel, 55 mg of nine-water nitrate chromium, 30 mg of glycene, 125 mg of urea, 13 mg of polyvinyl alcohol, and 20 ml of ultrapure water, magnetic force for 20min, stir well Get solution A;
[0028] Step 3: Soak the foam nickel of the step in step two in the solution A obtained in step two, transferred to the hydrothermal reaction kettle of the counterpinphenylphenylene and sealed, followed by fixing the inner liner in the outer extrapyl in the ...
Embodiment 2
[0035] The preparation method of the preparation of a chromium-doped nickel-based hydroxide self-supporting electrode of the present invention, and the specific steps include:
[0036] Step 1: Pretreatment of foam nickel. The foamed nickel was cut into a 1 cm × 5 cm block, and it washed it in acetone solution for 15 min, then poured into a 1 mol / L hydrochloric acid solution to ultrasonically cleaning 10 min, and finally washed with anhydrous ethanol and ultrapure water. 2 times, then drying at 35 ° C for 10 hours;
[0037] Step 2: 180 mg of hexahydrate nickel nickel, 65 mg of nine-water chromium, 20 mg of vanadium, 115 mg of urea 14 mg of polyvinyl alcohol is mixed, and 21 ml of ultrapure water, magnetic force is stirred for 21 min, stir evenly Solution A;
[0038] Step 3: Soak the foam nickel of the step in step two in the solution A obtained in step two, transferred to the hydrothermal reaction kettle of the counterpinphenylphenylene and sealed, followed by fixing the inner li...
Embodiment 3
[0041] The preparation method of the preparation of a chromium-doped nickel-based hydroxide self-supporting electrode of the present invention, and the specific steps include:
[0042] Step 1: Pretreatment of foam nickel. The foamed nickel was cut into a block of 1 cm × 5 cm, and it washed it in acetone solution for 11 min, then poured into a mixed 2 mol / L hydrochloric acid solution for 8 minutes, and finally washed with anhydrous ethanol and ultrapure water. 3 times, then dry at 30 ° C drying 11h after 11h;
[0043] Step 2: 175 mg of hexahydrate nickel, 60 mg of nine-water nitrate chromium, 25 mg of vanadium chloride, 120 mg of urea, 15 mg of polyvinyl alcohol, and 22 ml of ultrapure water, magnetic force for 22min, stirring evenly Get solution A;
[0044] Step 3: Soak the foam nickel of the steps obtained in step two, transferred to the hydrothermal reaction kettle of the counterpartenzene, followed by fixing the inner liner in an outer extract, filling The ratio was 62%, and th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com