Anti-influenza virus agent for suppressing aggravation of influenza
An influenza virus and anti-influenza technology, applied in antiviral agents, medical preparations containing active ingredients, pharmaceutical formulations, etc., can solve problems such as microbial influenza that have not yet been shown, and achieve the effect of inhibiting seriousness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061][Example 1] Preparation of OLB6378 concentrated powder
[0062]Bifidobacterium bifidum OLB6378 strain (accession number NITEBP-31) was anaerobic and cultured in a whey protein decomposition medium (a medium that uses enzymatically decomposed whey protein as the main nitrogen source). Obtain concentrated bacterial liquid. The concentrated bacterial solution was incubated at 80°C for more than 10 minutes, and then freeze-dried. The freeze-dried product obtained showed more than 2.5×1011Concentration of cells / g. The thus prepared freeze-dried product of the heat-treated OLB6378 strain was called OLB6378 concentrated powder, and the anti-influenza virus effect was tested as described later.
Embodiment 2
[0063][Example 2] Evaluation of the anti-influenza virus effect of OLB6378 concentrated powder
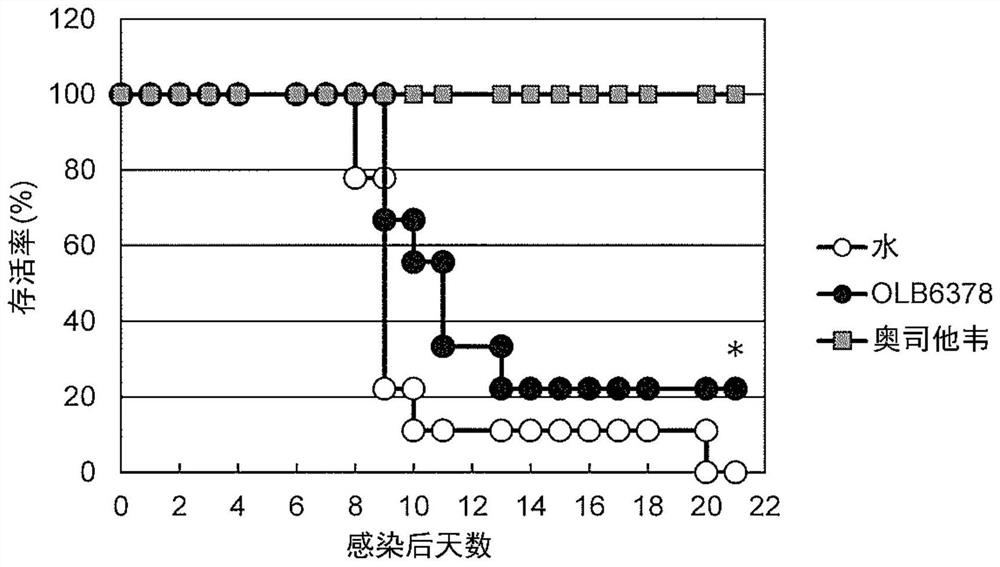
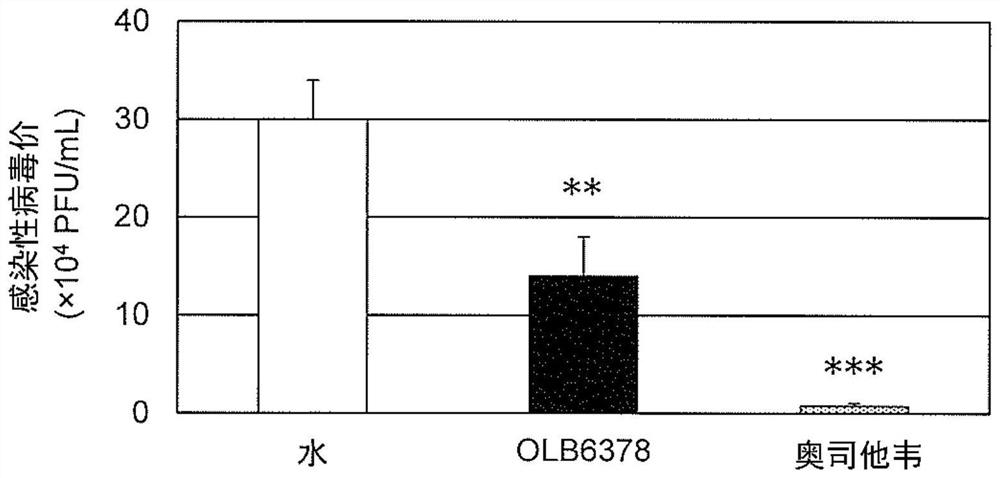
[0064]The anti-influenza virus effect of the OLB6378 concentrated powder prepared in Example 1 was evaluated in terms of "survival rate" after infection with influenza virus and "infectious viral value in respiratory organs (virus proliferation level)".
[0065]a) Survival rate
[0066]For BALB / c mice (female, 8 weeks old) (CLEA Japan, Inc.), water (water administration group) or OLB6378 concentrated powder was orally administered at a dose of 0.2 ml / person / day for 4 weeks 20% suspension (OLB6378 / Bifidobacterium administration group). At a time point 3 weeks after the start of oral administration, the anesthetic Somnopentyl(R)(5mg / kg body weight as the effective ingredient sodium pentobarbital) was administered intraperitoneally to anesthetize the mice, and the mouse domesticated influenza virus strain A / PR / 8 / 34 (H1N1) suspension was 20μl (2× LD50[Equivalent to twice the amount of lethal dose], 0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com