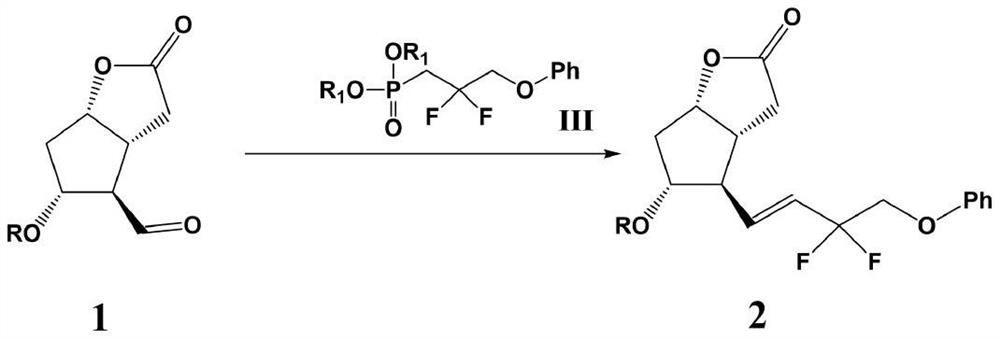
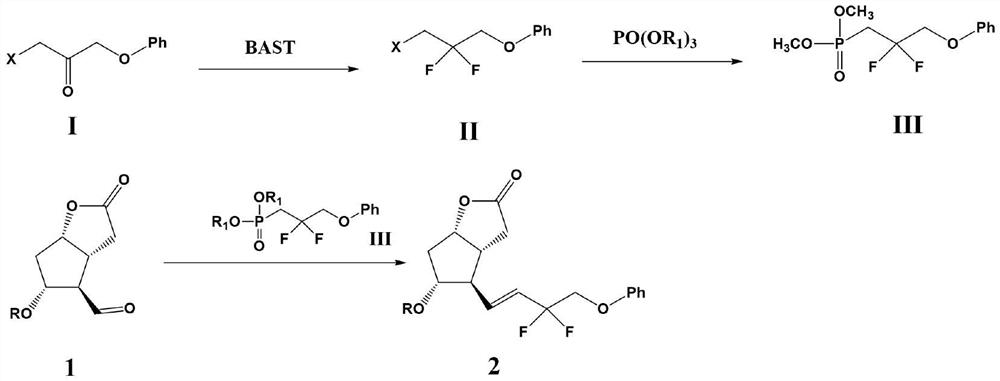
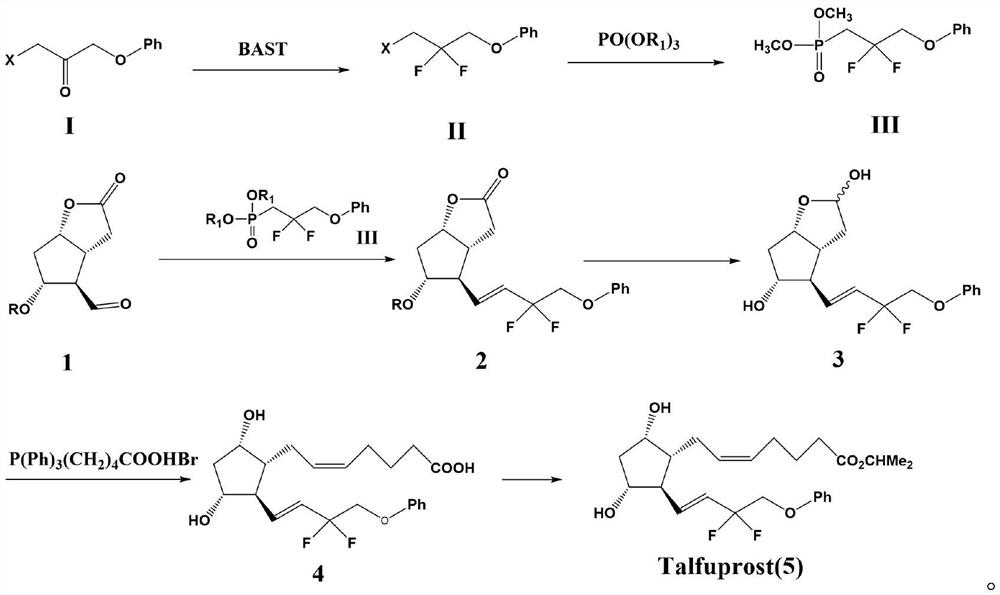
Preparation method of tafluprost
A technology of tafluprost and compound, applied in the field of medicinal chemistry, can solve the problems of low product purity, low yield of fluorination step, low conversion rate of target fluorinated product, etc., and achieve the effects of reducing preparation cost and improving economic benefit
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027]
[0028] 1-Bromo-3-phenoxypropan-2-one (15mmol) was dissolved in 100ml of dichloromethane, cooled to 0°C in an ice-water bath, BAST (5.6ml, about 30mmol) was slowly added, and the mixture was heated at 0 Stir for 1 hour at °C, then heat to 50°C and stir for an additional 5 hours. After the reaction was completed, cool to room temperature, slowly pour the reaction solution into a saturated sodium bicarbonate solution at 0°C, separate the organic phase and the aqueous phase, extract the aqueous phase once with dichloromethane, combine the organic phases, wash with saturated brine, and anhydrous After drying over sodium sulfate, the solvent was distilled off under reduced pressure, and recrystallized from ethanol to obtain 3.63 g of (3-bromo-2,2-difluoropropoxy)benzene with a yield of 96.5% and a purity of 98.9%. Mass spectrum m / z: theoretical value 249.9805; found value: 249.9833.
[0029] (3-Bromo-2,2-difluoropropoxy)benzene (10 mmol) and KI (11 mmol) were added to a...
Embodiment 2
[0033]
[0034](3-Bromo-2,2-difluoropropoxy)benzene (10 mmol) and KI (11 mmol) were added to a mixed solution of acetone (50 ml) and acetonitrile (50 ml) under nitrogen atmosphere. A solution of triethylphosphate (2ml, about 12mmol) in acetone (10ml) and acetonitrile (10ml) was slowly added dropwise at room temperature over 30 minutes. After dropping, the reaction mixture was stirred at room temperature for 15 hours, then filtered through celite on a glass filter, and the solvent was distilled off under reduced pressure, then dichloromethane (50ml) was added to dissolve, washed with saturated brine, and dried over anhydrous sodium sulfate. Afterwards, dichloromethane solution of (2,2-difluoro-3-phenoxypropyl) diethyl phosphate was obtained and set aside.
[0035] Sodium hydride (60% dispersion in mineral oil, 12 mmol) was dispersed in tetrahydrofuran (20 mL) under a nitrogen atmosphere to form a suspension, cooled to 0° C. in an ice-water bath, and the (2,2- Difluoro-3-phe...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap