Application of valeric acid derivatives in treatment of hereditary cerebellar ataxia
An ataxia, hereditary technology, applied in the prevention and improvement of hereditary cerebellar ataxia, the application of valeric acid derivatives in the treatment, autosomal recessive spinocerebellar ataxia 20, can solve the mechanism of SCAR20 Unclear, effective drug treatment is impossible to talk about, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
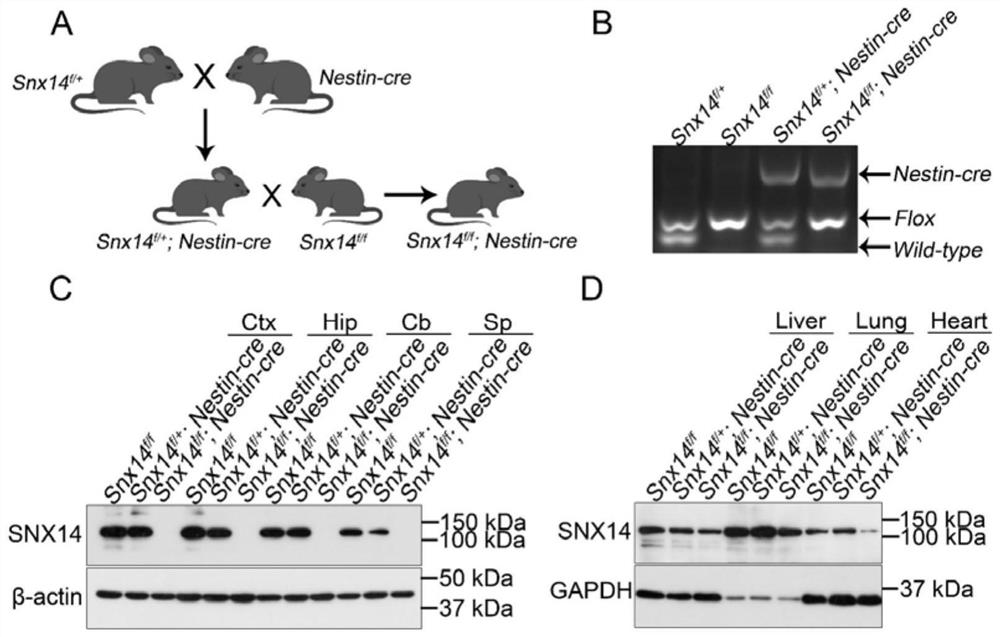
[0105] Example 1. Construction and Identification of Conditional Knockout Mice in SNX14 Brain
[0106] Animals used in the experiment were used to construct SNX14 brain condition knockout mice using the Loxp-Cre system. f / f Nestin-CRE mice. Transgenic SNX14 involved flox / + (SNX14 f / + The mouse commissioning industry (Guangzhou) company was constructed, B6. CG-TG (NES-CRE) 1 kln / j (Nestin-Cre) mouse was purchased from Jackson Lab. First of all, SNX14 f / + Get SNX14 with Nestin-Cre f / + Nestin-cre mice, the latter is again with SNX14 f / f Matches to get the category knockout mouse SNX14 f / f Nestin-Cre figure 1 A), I can get SNX14 f / + SNX14 f / f SNX14 f / + Nestin-CRE mice. Four genotype mice can be identified by genotype identification of the child. figure 1 B). Immunoprotice verification, SNX14 f / f Nestin-Cre mouse central nervous system's brain regions did not express SNX14 ( figure 1 C) The peripheral administrator is expressed ( figure 1 D), indicating that the SNX14 gene is onl...
Embodiment 2
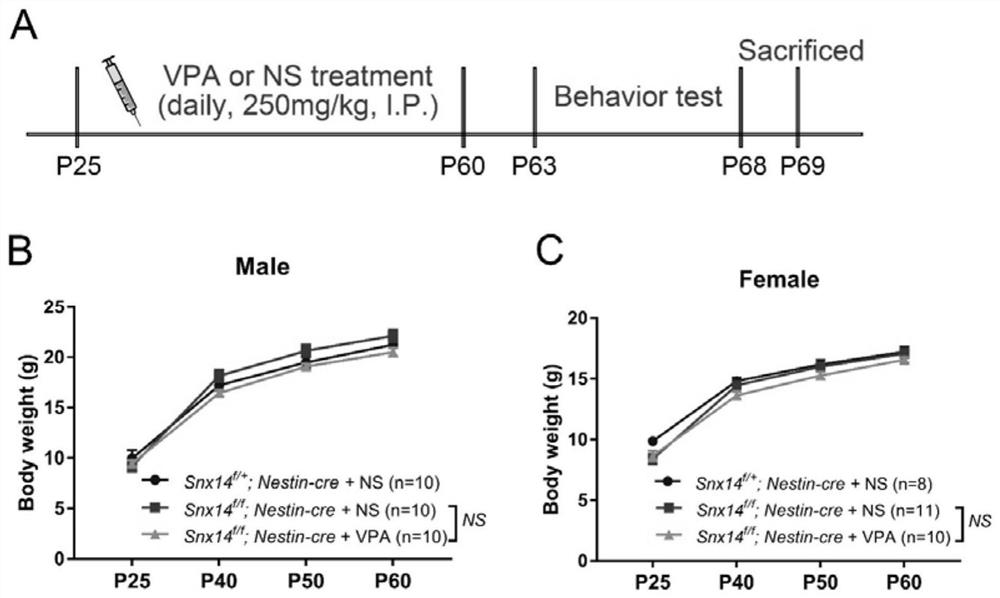
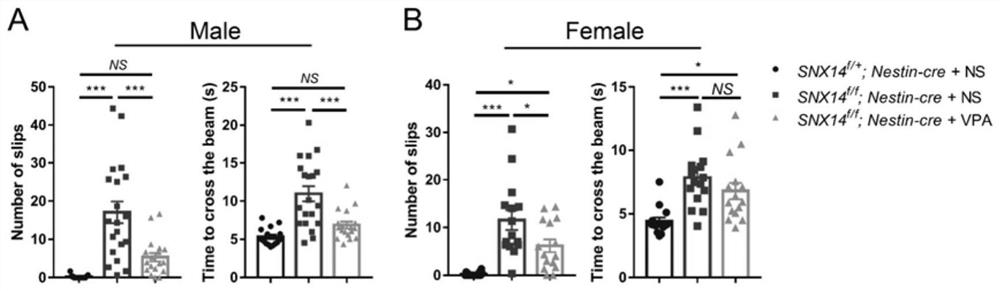
[0107] Example 2. Experimental process of VPA administration
[0108] The drug VPA was purchased from Sigma-Aldrich (P4543), with a 20 mg / kg spare with physiological saline (NS). Experiments are divided into three groups: SNX14 f / + ; Nestin-Cre + NS group, SNX14 f / f Nestin-Cre + NS group and SNX14 f / f Nestin-CRE + VPA group. Mice were administered from P25 days to receive abdominal injection VPA (250 mg / kg body weight / day) for 35 days, and the control group had a volume of NS ( figure 2 A). Three days (P63) after the end of the administration, behavioral tests were started. After the test was completed, the mice were killed, and the brain was subsequently experiment.
Embodiment 3
[0109] Example 3. VPA treatment has no significant effect on the body weight of mice.
[0110] See figure 2 B and 2C, during treatment (P25, P40, P50 and P60), SNX14 f / f Nestin-Cre + VPA group of mole weight and SNX14 f / f Nestin-Cre + NS groups have no significant differences, and females are also significantly different. It is suggested that the long-term VPA treatment has no significant adverse effect on the physical condition of the male and female. The data is indicated by mean ± standard mistake (Mean ± SEM), which was statistically analyzed by TW-WAY ANOVA, with 8-11 mice. NS representatives have no significant difference.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com