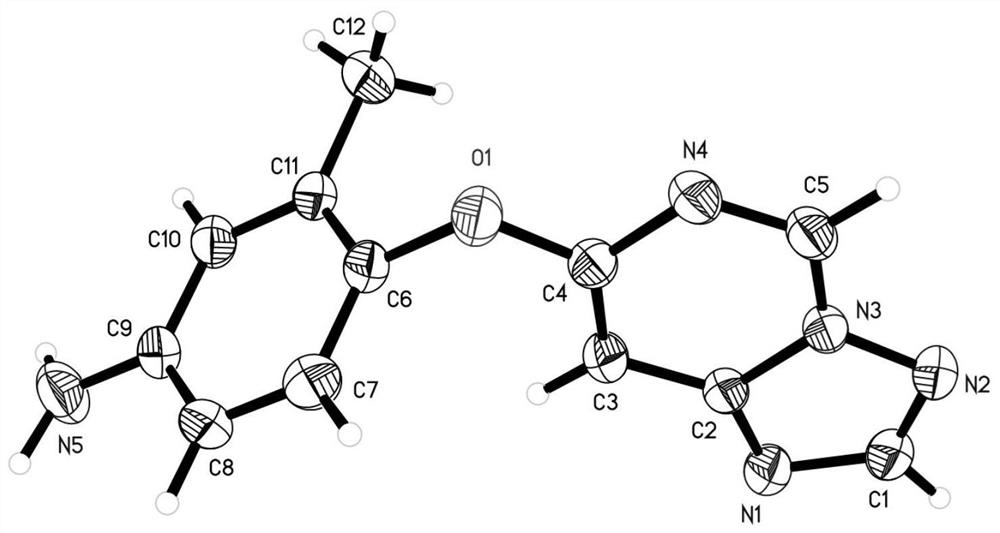
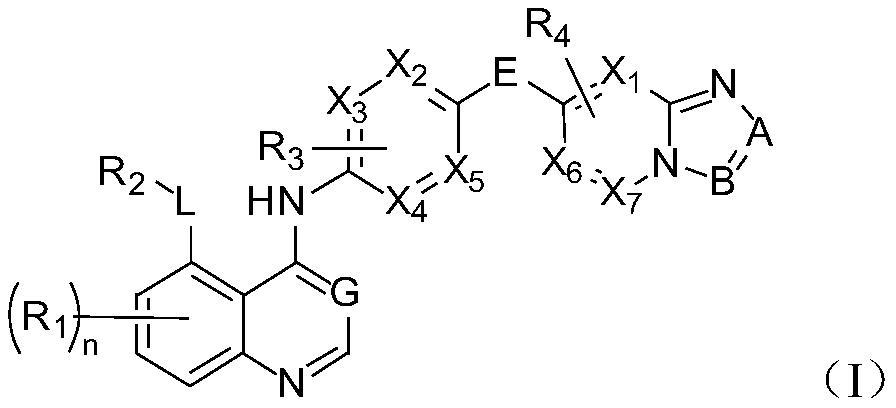
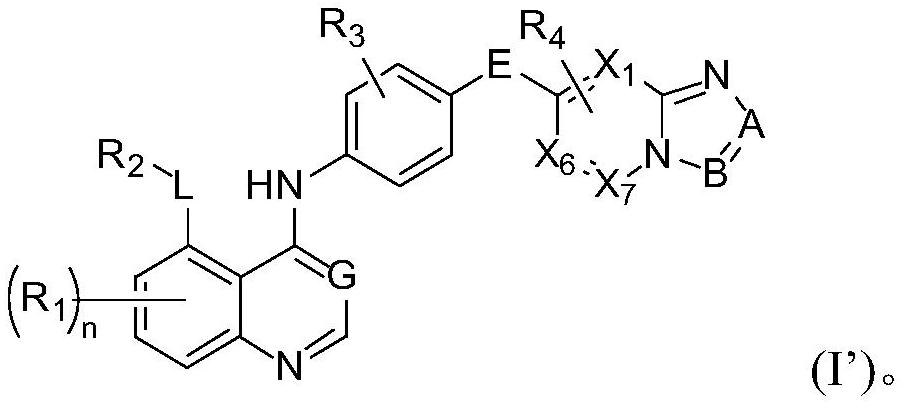
Quinazoline derivatives as antitumor agents
A compound and pharmaceutical technology, applied in the field of novel quinazoline compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0463] N-(4-([1,2,4]triazol[4,3-c]pyrimidin-7-yloxy)-3-methylphenyl)-5-(3-(dimethylamino) Azetidin-1-yl)-6-methoxyquinazolin-4-amine
[0464]
[0465] Step 1: 4-Chloro-6-hydrazinopyrimidine
[0466]
[0467] To a solution of 4,6-dichloropyrimidine (100 g, 675.7 mmol) in EtOH (900 mL) was added 50 wt% aqueous hydrazine (130 mL) dropwise at 45°C over 2 hours. The reaction was then stirred at 45-50°C for 2 hours. The crude mixture was filtered and the solid was washed with water to give the desired product (91 g, 94%) as a yellow solid. MS(ESI)m / z:145.1(M+H) + .
[0468] Step 2: 7-Chloro-[1,2,4]triazolo[1,5-c]pyrimidine
[0469]
[0470] 4-Chloro-6-hydrazinopyrimidine (91 g, 632 mmol) was dissolved in HC (OMe) 3 The solution in was stirred overnight at 90 °C. The crude mixture was concentrated and washed with NaHCO 3 Dilute with aqueous solution (500 mL). The resulting mixture was extracted with EtOAc (500 mL×2). The organic phase was washed with water and brin...
Embodiment 2
[0513] (R)-N-(4-([1,2,4]triazol[1,5-c]pyrimidin-7-yloxy)-3-methylphenyl)-5-(3-(di Methylamino)pyrrolidin-1-yl)-6-methoxyquinazolin-4-amine
[0514]
[0515] The title compound was prepared using a method similar to Example 1 to afford the desired product as a white solid. MS:m / z512(M+H) + . 1 H NMR (400MHz, CDCl 3 )δ13.66(d, J=24.1Hz, 1H), 9.20(dd, J=3.6, 1.2Hz, 1H), 8.57(s, 1H), 8.32(d, J=4.1Hz, 1H), 8.06- 7.63(m,4H),7.49(dd,J=9.2,4.3Hz,1H),7.12(dd,J=13.5,8.7Hz,1H),6.88(dd,J=16.3,1.3Hz,1H),4.01 (d, J=3.7Hz, 3H), 3.69-3.24(m, 4H), 3.02(d, J=33.0Hz, 1H), 2.44-2.20(m, 11H).
Embodiment 3
[0517] (S)-N-(4-([1,2,4]triazol[1,5-c]pyrimidin-7-yloxy)-3-methylphenyl)-5-(3-(two Methylamino)pyrrolidin-1-yl)-6-methoxyquinazolin-4-amine
[0518]
[0519] The title compound was prepared using a method similar to Example 1 to give the desired product as a yellow solid. MS:512(M+H) + . 1 H NMR (400MHz, CDCl 3 )δ13.68(d, J=23.0Hz, 1H), 9.20(d, J=3.6Hz, 1H), 8.57(s, 1H), 8.32(d, J=4.0Hz, 1H), 8.07-7.63( m,4H),7.48(dd,J=9.2,4.0Hz,1H),7.11(dd,J=13.9,8.7Hz,1H),6.88(d,J=16.3Hz,1H),4.01(d,J =2.7Hz, 3H), 3.70-3.22(m, 4H), 3.12-2.89(m, 1H), 2.59-2.03(m, 11H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com