Urate oxidase with catalytic activity
A technology of urate oxidase and urate oxidase protein, which is applied in the biological field and can solve the problems of low immunogenicity and in vivo stability of urate oxidase protein
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1: Construction of rhUOX-N mutants (resurrected human-source uricase with mutants, on the basis of human uricase pseudogene, an active uricase protein obtained by mutation of amino acid sites, N in rhUOX-N refers to The number of amino acid positions where site-directed mutagenesis is completed).
[0039] It should be noted that the construction process of the mutant rhUOX-N in this experiment is based on the gene of the previous mutant product rhUOX-(N-1) as a template, using overlap extension PCR technology, and site-directed mutation of 17 amino acid sites that have not yet been identified. A certain site of the mutation was verified by positive screening, and then the mutant rhUOX-N was obtained. And the mutant rhUOX-N is also the site-directed mutation template for the next mutant rhUOX-(N+1).
[0040] Obtain the plasmid template: inoculate the strain of plasmid pET-22b(+) / rhUOX-(N-1) containing the previous successfully mutated amino acid site in LB liqui...
Embodiment 2
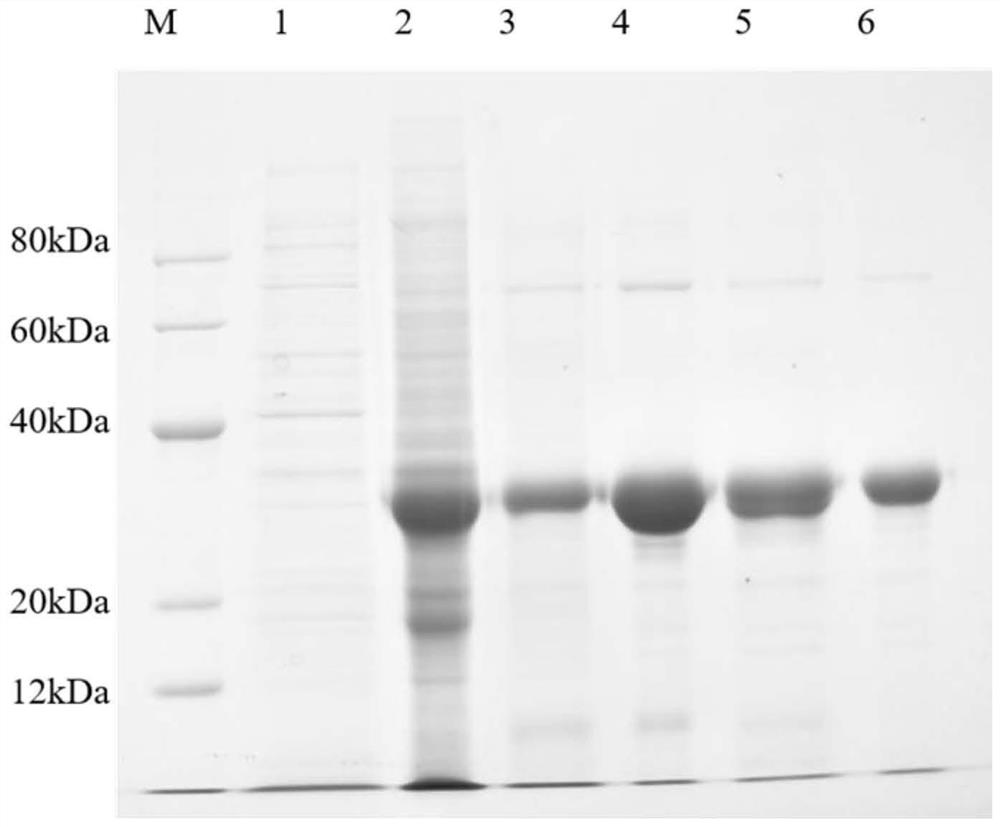
[0049] Example 2: Expression, separation and purification of rhUOX-17Mutant (resurrected human-source urate oxidase with 19 mutant of amino acid site, active uricase protein obtained by mutation of 17 amino acid sites on the basis of human uricase pseudogene)
[0050] Induced expression of rhUOX-17Mutant engineered strain: Inoculate the preserved rhUOX-17Mutant engineered strain into liquid LB medium containing 100 μg / mL, culture on a shaker at 200 rpm at 37°C for 11 hours, then transfer to fresh fermentation medium to continue After culturing for 4 hours, 0.2 mM IPTG was added, continued culturing for 5 hours, and the fermentation broth was collected.
[0051] Bacterial collection by centrifugation: Collect the fermented rhUOX-17Mutant engineering strain by centrifugation at 4°C at 8000g for 20 minutes, resuspend the collected bacteria with purified water, and centrifuge under the same conditions to wash the collected bacteria.
[0052] Ultrasonic disruption of bacteria: Add ...
Embodiment 3
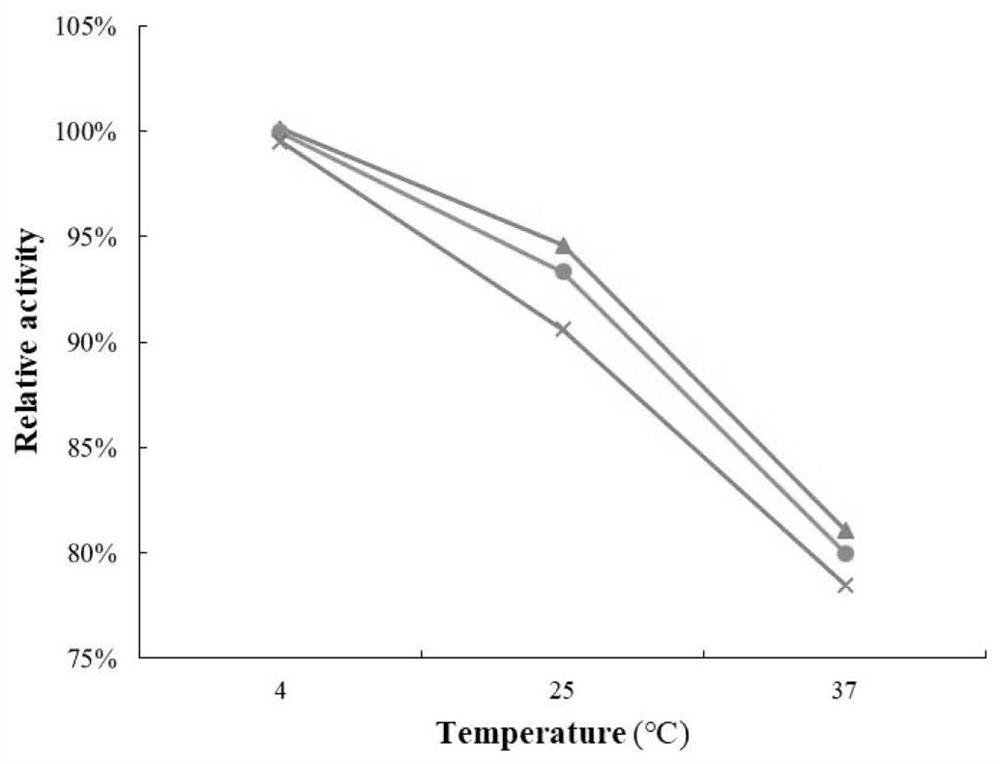
[0058] Example 3: Analysis of specific activity and enzymatic properties of rhUOX-17Mutant
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap