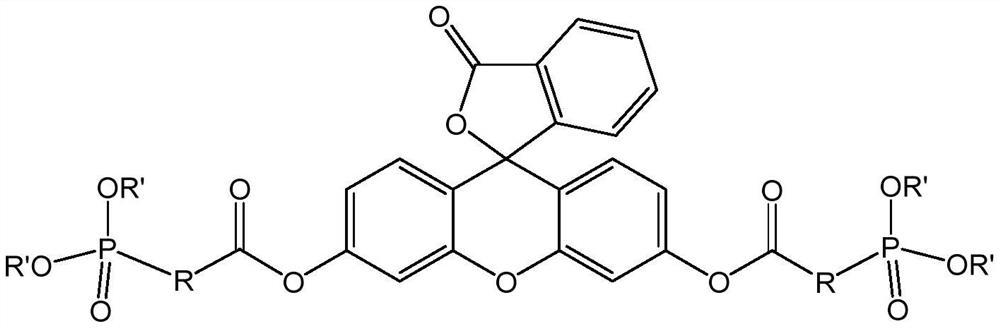
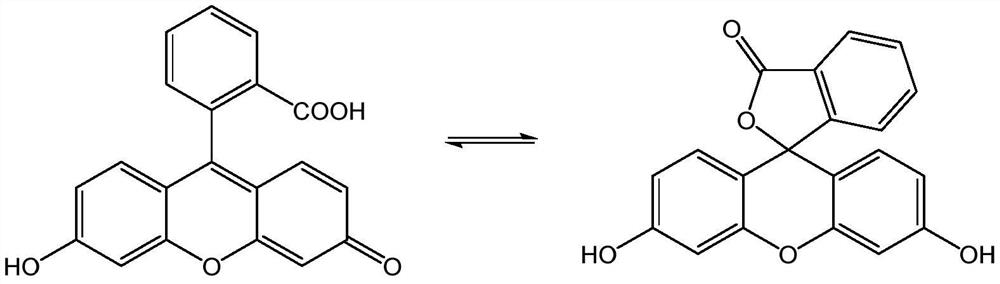
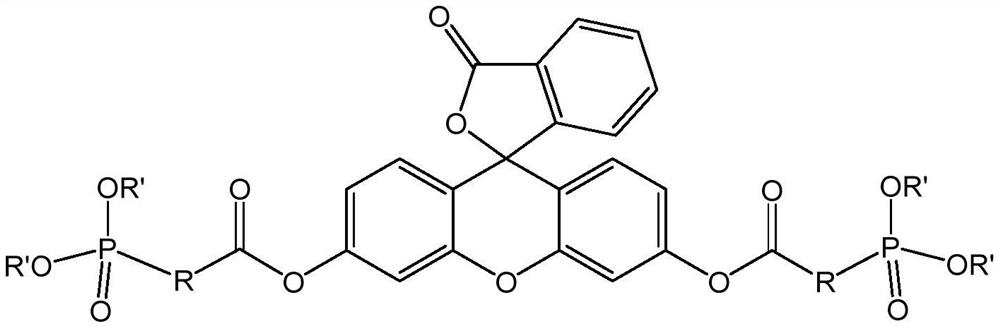
A kind of lipophilic fluorescein probe and preparation method thereof
A fluorescein and lipophilic technology, applied in fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., to achieve high affinity, not easy to self-hydrolyze, and easy to operate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] The following methods were used to prepare lipophilic fluorescein probes according to the present application:
[0056]
[0057] (1) slowly add the oxalyl chloride of 35g in the 5-bromo-4-methylvaleric acid of 10g, then react at room temperature for 5 hours, after the completion of the reaction, under reduced pressure distillation at 80 ℃, obtain the product 5-bromo- of 10.33g- 4-Methylvaleryl chloride;
[0058] (2) 17.68g (0.05321mol) of fluorescein was added to 110mL (145.8g) of dichloromethane, the 5-bromo-4-methylvaleryl chloride obtained in step (1) was slowly added under stirring at room temperature, and then heated to reflux The reaction was performed for 5 hours, washed with saturated aqueous sodium bicarbonate solution after the reaction was completed, and the organic phase was distilled under reduced pressure at 40° C. The residue was washed with ethanol, and then dried at 55° C. for 3 hours to obtain 28.08g of product two ( 5-Bromo-4-methylvaleric acid) f...
Embodiment 2
[0061] The following methods were used to prepare lipophilic fluorescein probes according to the present application:
[0062]
[0063] (1) slowly add the oxalyl chloride of 30g in the 6-bromohexanoic acid of 10g, then react at room temperature for 3.5 hours, after the completion of the reaction, under reduced pressure distillation at 75 ℃, obtain the product 6-bromohexanoyl chloride of 10.11g;
[0064] (2) 19.67 g (0.05919 mol) of fluorescein was added to 140 mL (185.5 g) of dichloromethane, the 6-bromohexanoyl chloride obtained in step (1) was slowly added under stirring at room temperature, and then heated under reflux for 6 hours to react. After completion, it was washed with saturated aqueous sodium bicarbonate solution, and the layers were separated. The organic phase was distilled under reduced pressure at 45 °C, the residue was washed with methanol, and then dried at 55 °C for 3.5 hours to obtain 26.27 g of the product bis(6-bromohexanoic acid) ) fluorescein ester; ...
Embodiment 3
[0067] The following methods were used to prepare lipophilic fluorescein probes according to the present application:
[0068]
[0069] (1) slowly add the thionyl chloride of 40g to the 6-bromo-5-methylhexanoic acid of 10g, then react at room temperature for 2 hours, after the completion of the reaction, distillation under reduced pressure at 70° C. to obtain 10.41g of product 6- bromo-5-methylhexanoyl chloride;
[0070] (2) 15.96g (0.04803mol) of fluorescein was added to 100mL (132.5g) of dichloromethane, the 6-bromo-5-methylhexanoyl chloride obtained in step (1) was slowly added under stirring at room temperature, and then heated to reflux The reaction was carried out for 2 hours, washed with saturated aqueous sodium bicarbonate solution after the reaction was completed, and the organic phase was distilled under reduced pressure at 45° C. The residue was washed with methanol, and then dried at 60° C. for 4 hours to obtain 26.71 g of product two ( 6-Bromo-5-methylhexanoic...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap