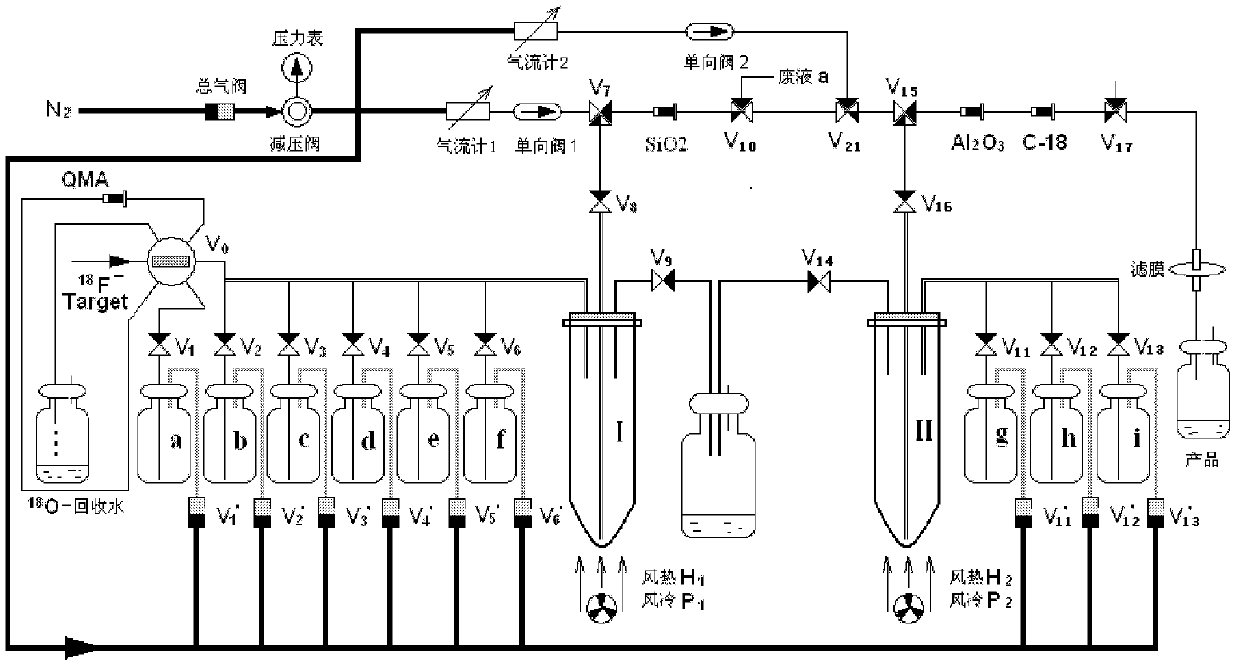
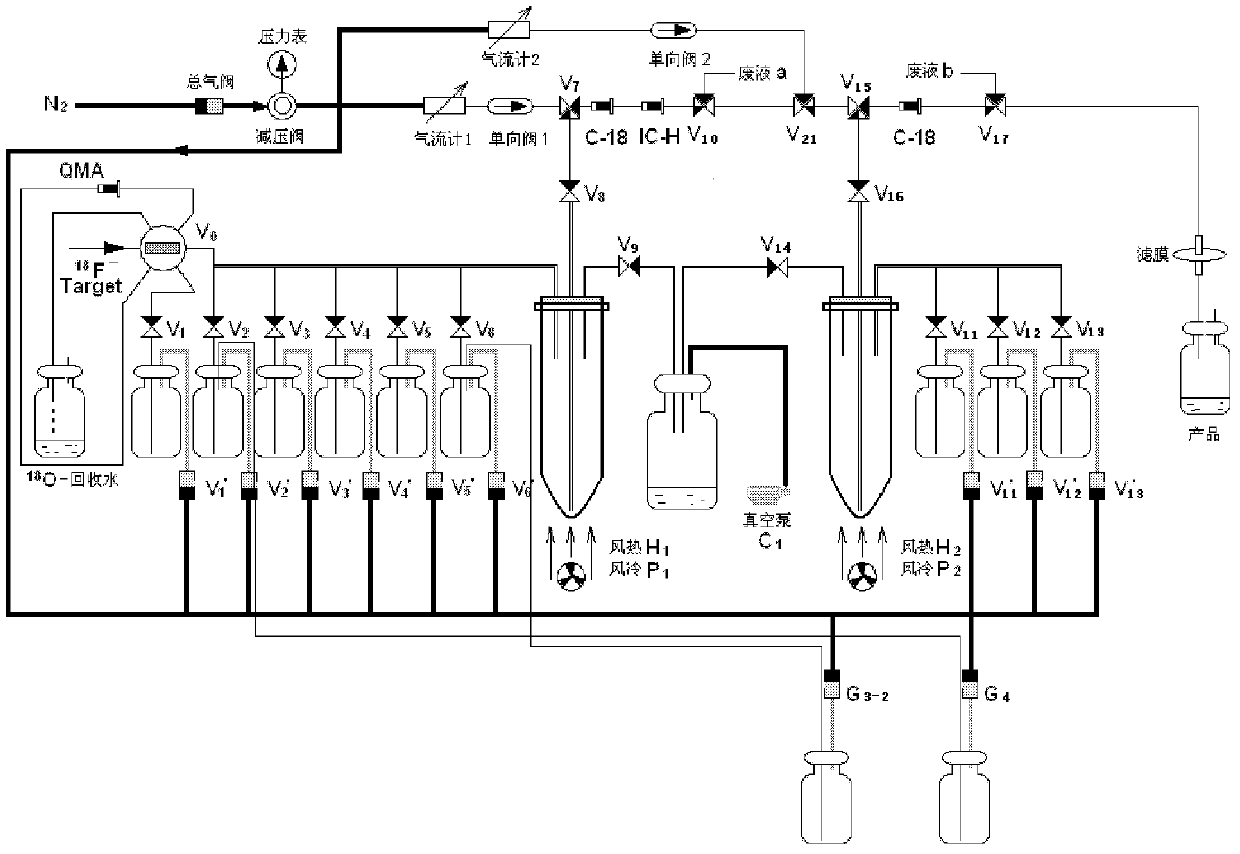
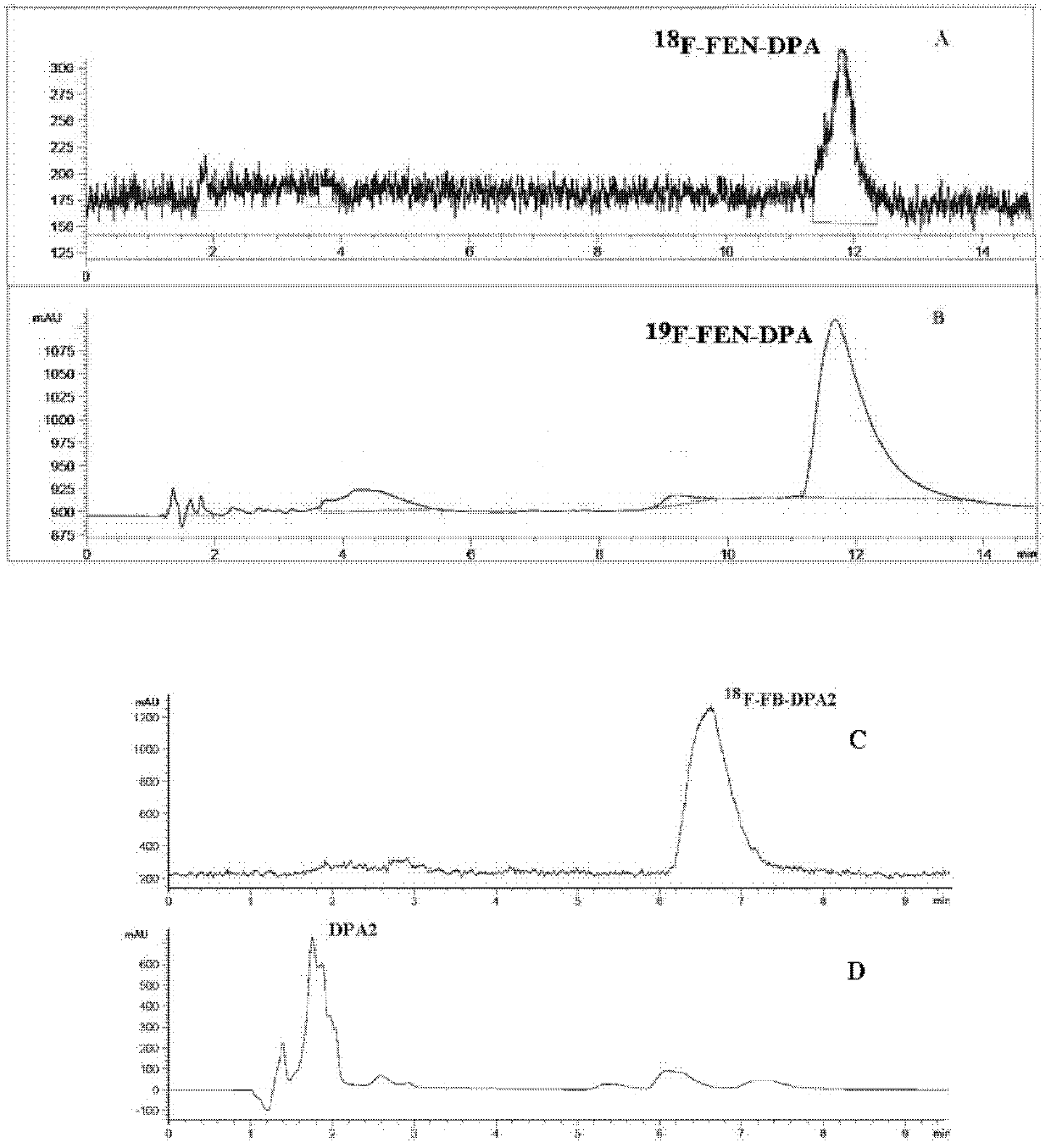
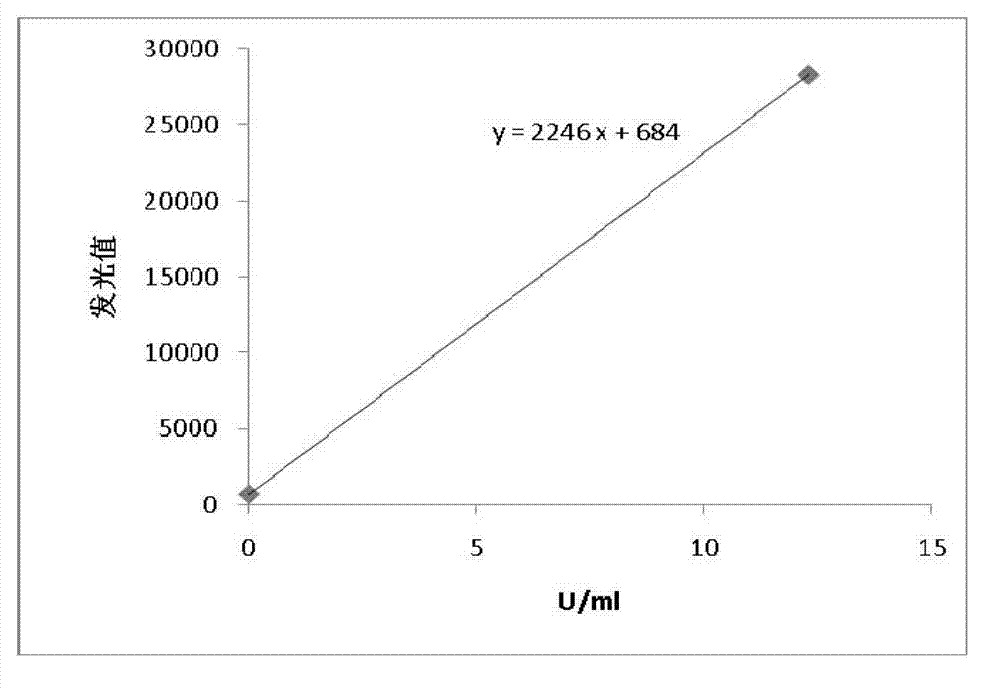
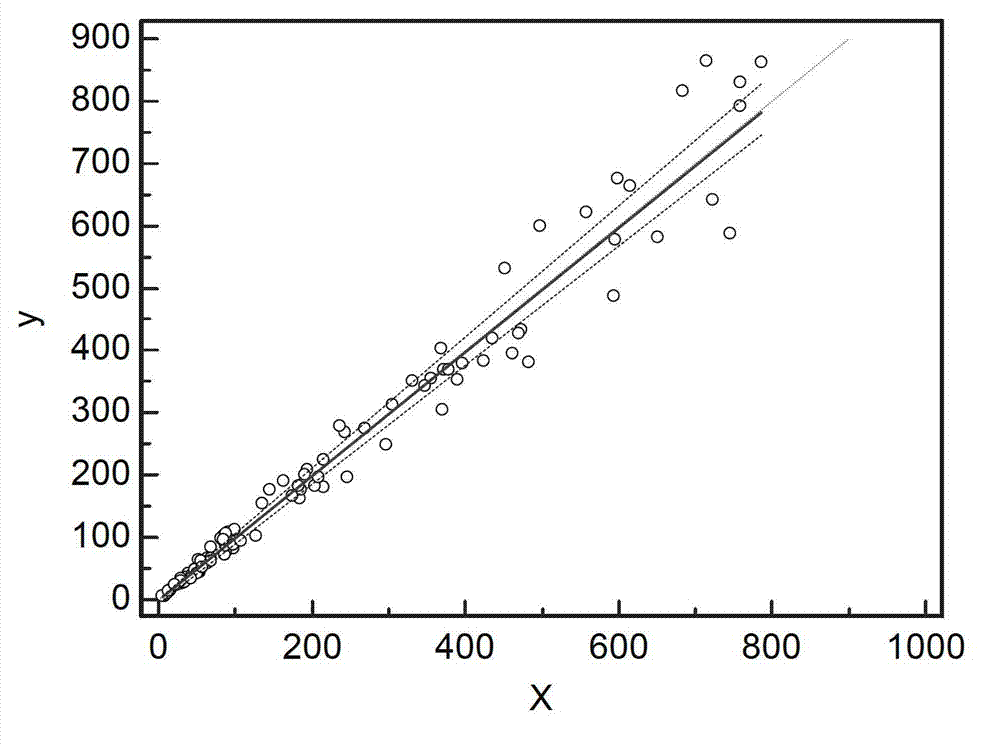
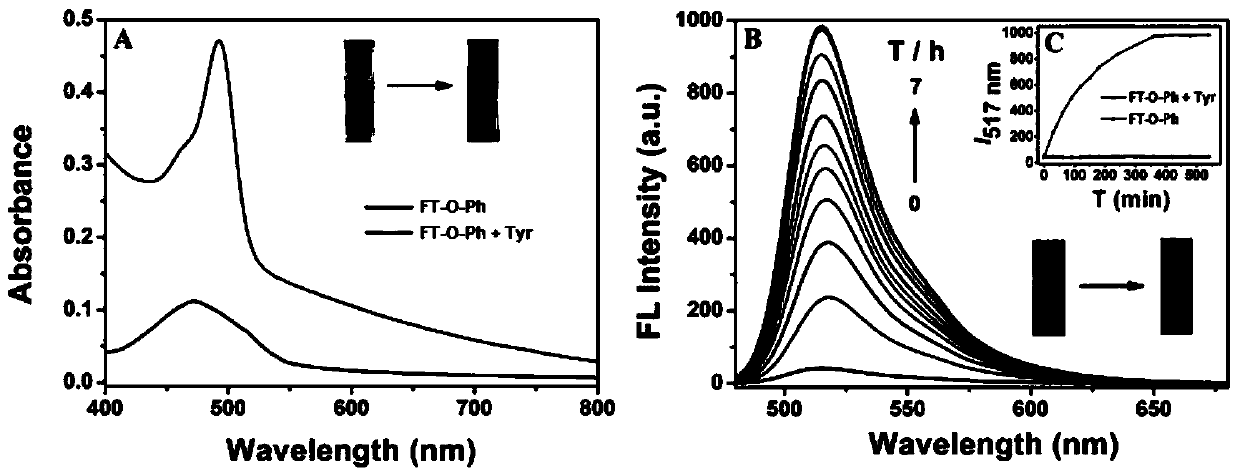
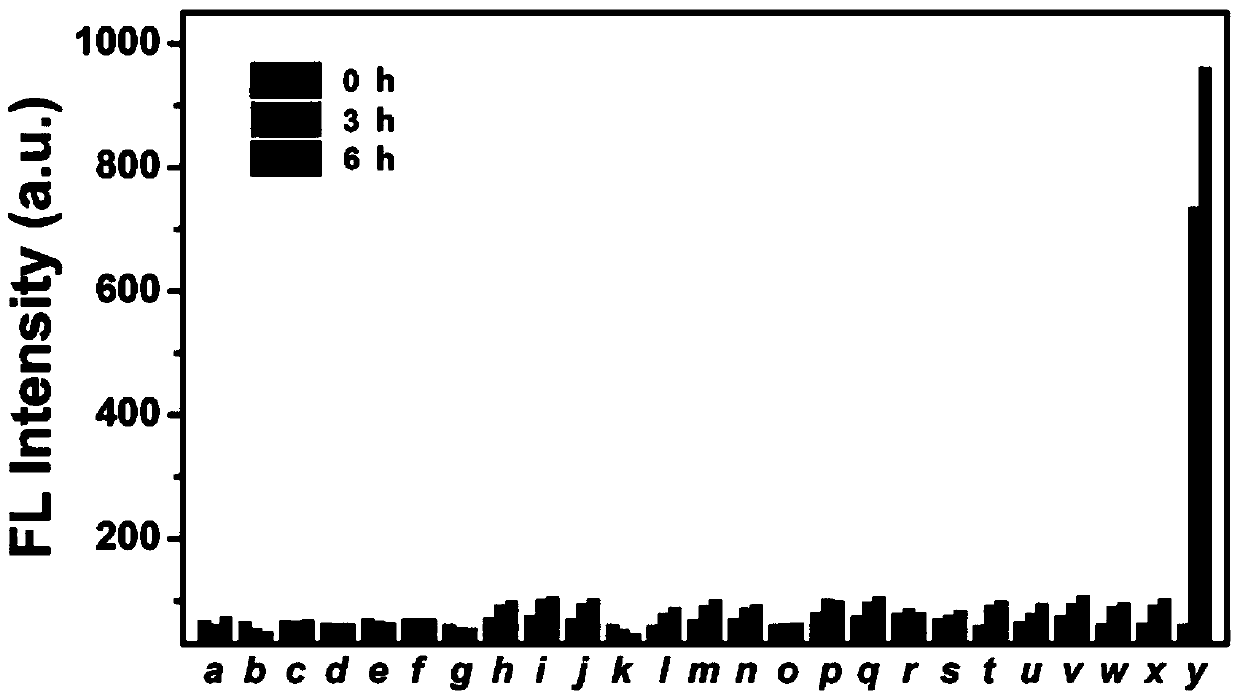
Patents
Literature
75 results about "Fluoreszein" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
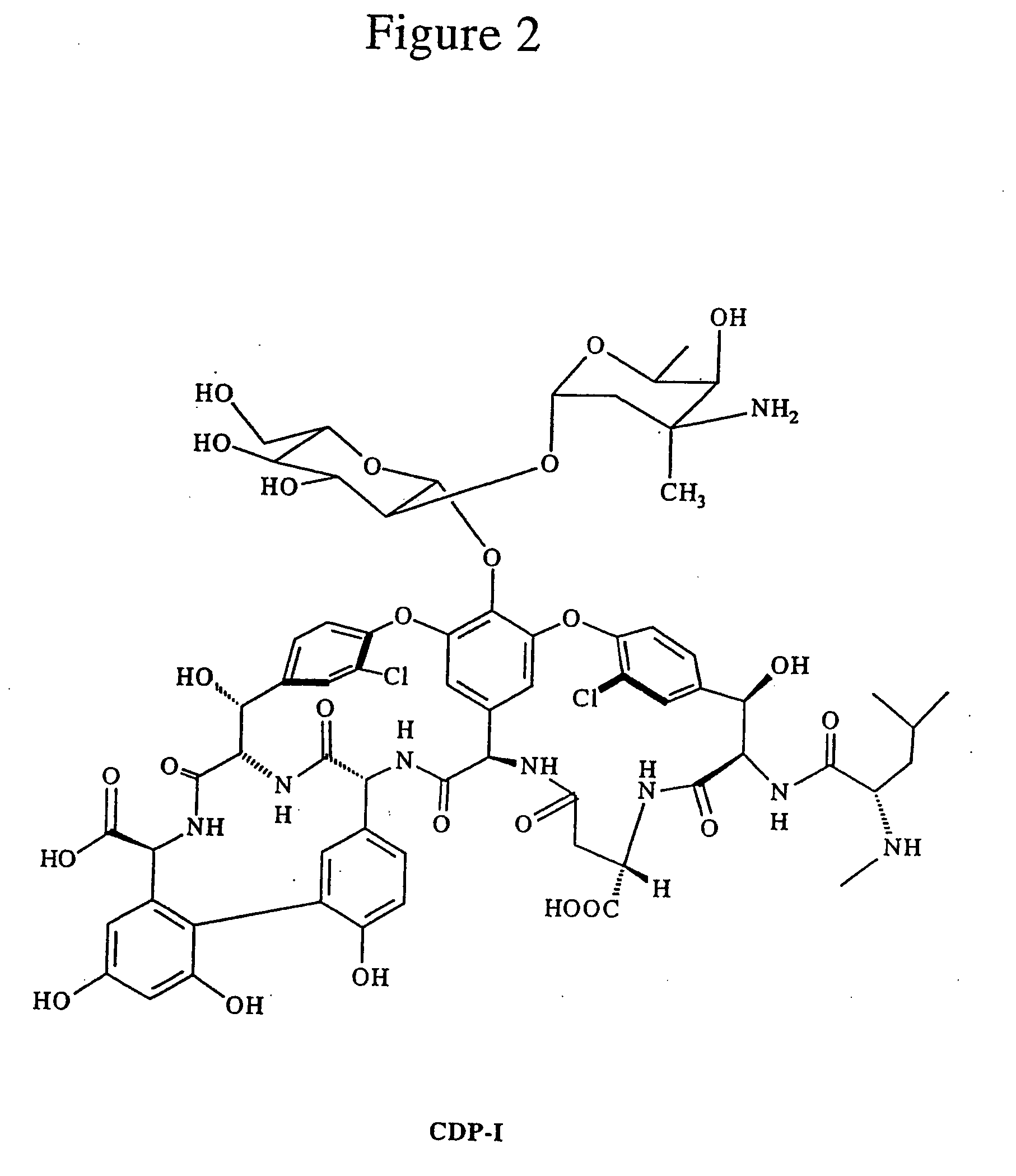
Polyamine micromolecular developer, production method and application thereof
InactiveCN102442996AReduce intakeExcellent treatment monitoringOrganic chemistryIn-vivo radioactive preparationsAlkaneApoptosis
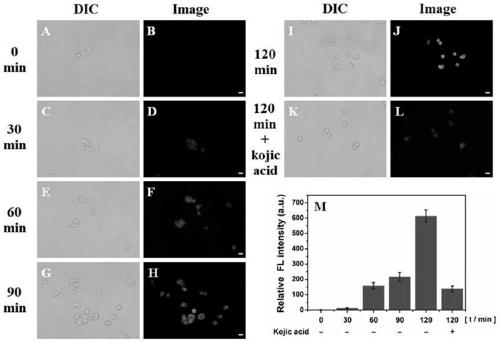
The invention relates to a polyamine micromolecular compound, comprising the following structures described in the specification, wherein M<x+> is 0, Zn<2+>, Ga<3+>, Gd<3+>, Ca<2+> or other divalent metal ions and trivalent metal ions; S is a reporter group (including a nuclide labeling prothetic group, a paramagnet, a fluorescein or a microvesicle), such as the nuclide labeling prothetic group: -<11>CH3, -CH2CH2<18>F, -CH2CH2(OCH2CH2)2NHCOC6H4<18>F-p or -CH2CH2(OCH2CH2)2NH-CH2CH2<18>F; R is -H, -OCH3, -OCH2CH3 or -Cl; and R1, R2, R3 and R4 are hydrogen, carboxyl, alkane, alkylene or heteroalkyl. The invention further relates to application of the compound in preparing cell death or apoptosis developers of target phosphatidyl serine (PS) and / or apoptotic cell early free Zn<2+>. The compound provided by the invention is a specific multiamine micromolecular developer for target phosphatidyl serine (PS) and / or apoptotic cell early free Zn<2+>, which can be used for monitoring curative effects of PS-related anti-tumor chemotherapy, radiotherapy, biological therapy and the like; the compound can be used for early differential diagnosis of neurodegenerative diseases (senile dementia and Parkinson's disease), cerebral apoplexy, AIDS (Acquired immune deficiency syndrome), thrombus, atheromatous plaque and myocardial infarction; and the compound can also be used for differential diagnosis and curative effect monitoring of other diseases related to PS expression in the cell death or apoptosis process, such as inflammation development and anti-inflammation therapeutic development.
Owner:THE FIRST AFFILIATED HOSPITAL OF SUN YAT SEN UNIV
Immunofluorescence dipstick component for quickly and quantitatively detecting protein of plurality of types and detection card component prepared from same and preparation method thereof
ActiveCN102662065AHigh dilution factorReduce matrix effectBiological testingCreatine kinaseCreatine kinase isoenzyme
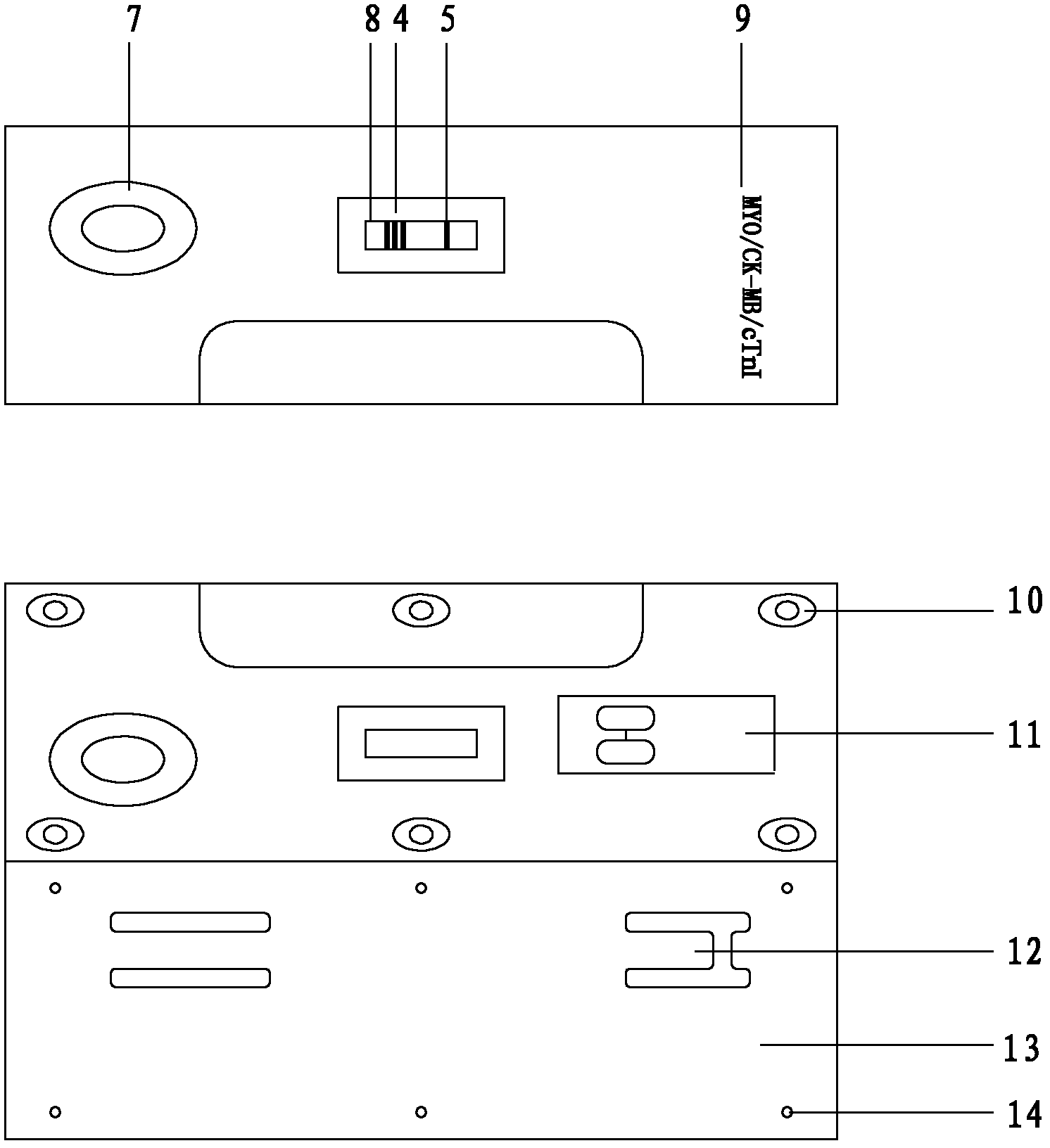
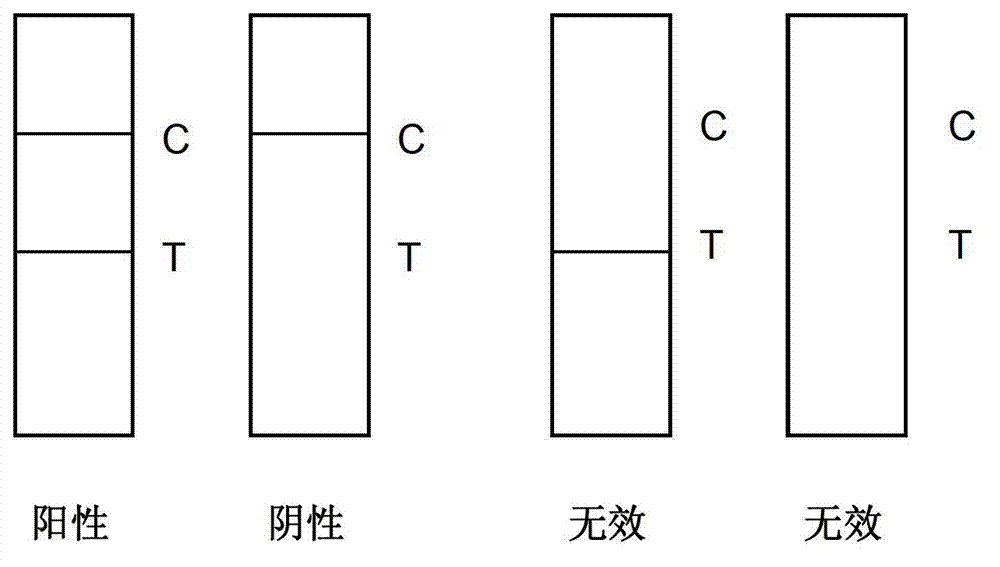
The invention discloses an immunofluorescence dipstick component for quickly and quantitatively detecting protein of a plurality of types and a detection card component prepared from the same and a preparation method thereof, wherein the protein of a plurality of types comprises muscle hemoglobin / creatine kinase isoenzyme / troponin I; the dipstick component comprises a dipstick consisting of a bottom liner, a water-absorbing pad, a coated analysis membrane and a sample pad and independently packaged fluorescein mark specific antibodies; three detection lines and a quality control line are arranged on the coated analysis membrane; the detection line on the coated analysis membrane is respectively coated with an anti-myoglobin monoclonal antibody line, an anti-creatine kinase isozyme monoclonal antibody line and a troponin I monoclonal antibody line; the quality control line is coated with a rabbit IgG antibody; the fluorescein mark specific antibodies comprise the anti-myoglobin monoclonal antibody line, the anti-creatine kinase isozyme monoclonal antibody line, an troponin I monoclonal antibody and an anti-rabbit IgG antibody; and the detection card component comprises the dipstick, a card box consisting of a cover plate and a back plate and independently packaged platinum porphyrin mark specific antibodies and can synchronously detect the muscle hemoglobin / creatine kinase isoenzyme / troponin I, is simple to operate, is quick and sensitive, and has good specificity.
Owner:GUANGZHOU HONGQI OPTICAL INSTR TECH
Genetic evaluation and suit method for individualized tumor therapy
InactiveCN102732600AImprove curative effectAvoid side effectsMicrobiological testing/measurementAnti-Tumor DrugsSide effect
The invention discloses a genetic evaluation and suit method for individualized tumor therapy. The genetic evaluation and package method includes steps of: 1) extracting a DNA sample of a subject; 2) carrying out target gene PCR amplification, purification, fragmentation and fluorescein labeling on the sample DNA; 3) carrying out gene chip hybridization on fluorescein labeled PCR products to detect SNP sites of the target gene; and 4) analyzing curative effect and toxicity of antitumor drug on the subject, according to the genetic typing results obtained in the step 3). Therapeutic effect evaluation of tumor patient can be conducted through the related genetic typing, thus formulating the most scientific individualized therapy plan to improve efficacy, reduce or even avoid the toxic and side effects.
Owner:SHANG OUTDO BIOTECH CO LTD
Test paper used for detecting acute myocardial infarction, and preparation method and application method thereof
InactiveCN102901828AHigh sensitivityRealize specific quantitative detectionBiological testingSerum igeCreatine kinase
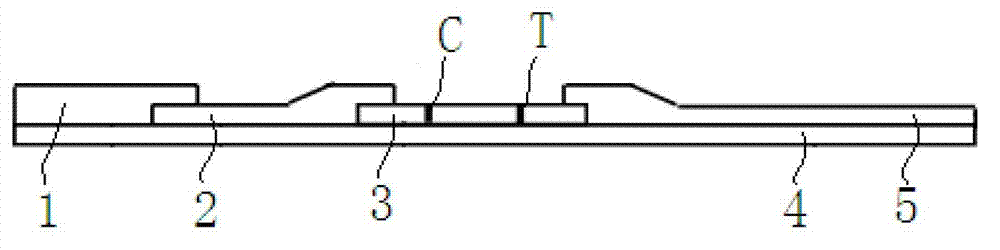
The invention provides a test paper used for detecting acute myocardial infarction, and a preparation method and an application method thereof. The test paper comprises a base plate and sequentially closely connected components adhered to the base plate, wherein the components are a sample absorption pad, a bonding pad, a chromatography membrane, and a water absorption pad. The bonding pad is coated with a glucan-antibody-fluorescein mixed marker. The chromatography membrane is provided with a detection band and a control band. A monoclonal antibody is fixed on the detection band. A rabbit anti-mouse IgG antibody which can be speficically bound with the glucan-antibody-fluorescein mixed marker is fixed on the control band. The sensitivity range of the reagent provided by the invention reaches 0.1ng / L. With the test paper, quantitative detections upon trace myoglobin, troponin I and creatine kinase isoenzyme in myocardial infarction patient serum can be realized. A result can be obtained in 3-5 minutes after sample spotting. The operation is simple, and professional operation is not needed.
Owner:武汉渊元医学科技有限公司
Substantially pure fluorescein
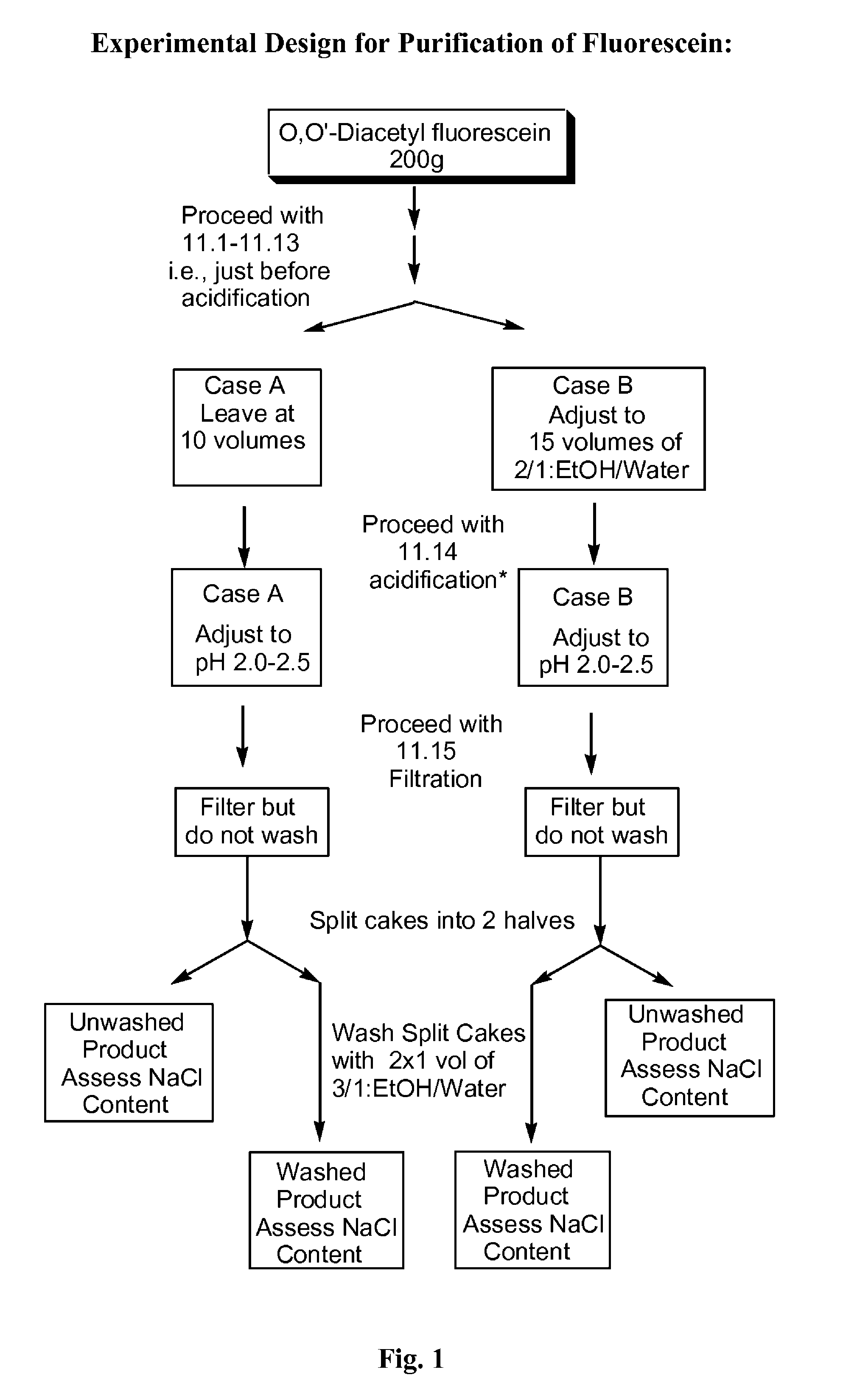
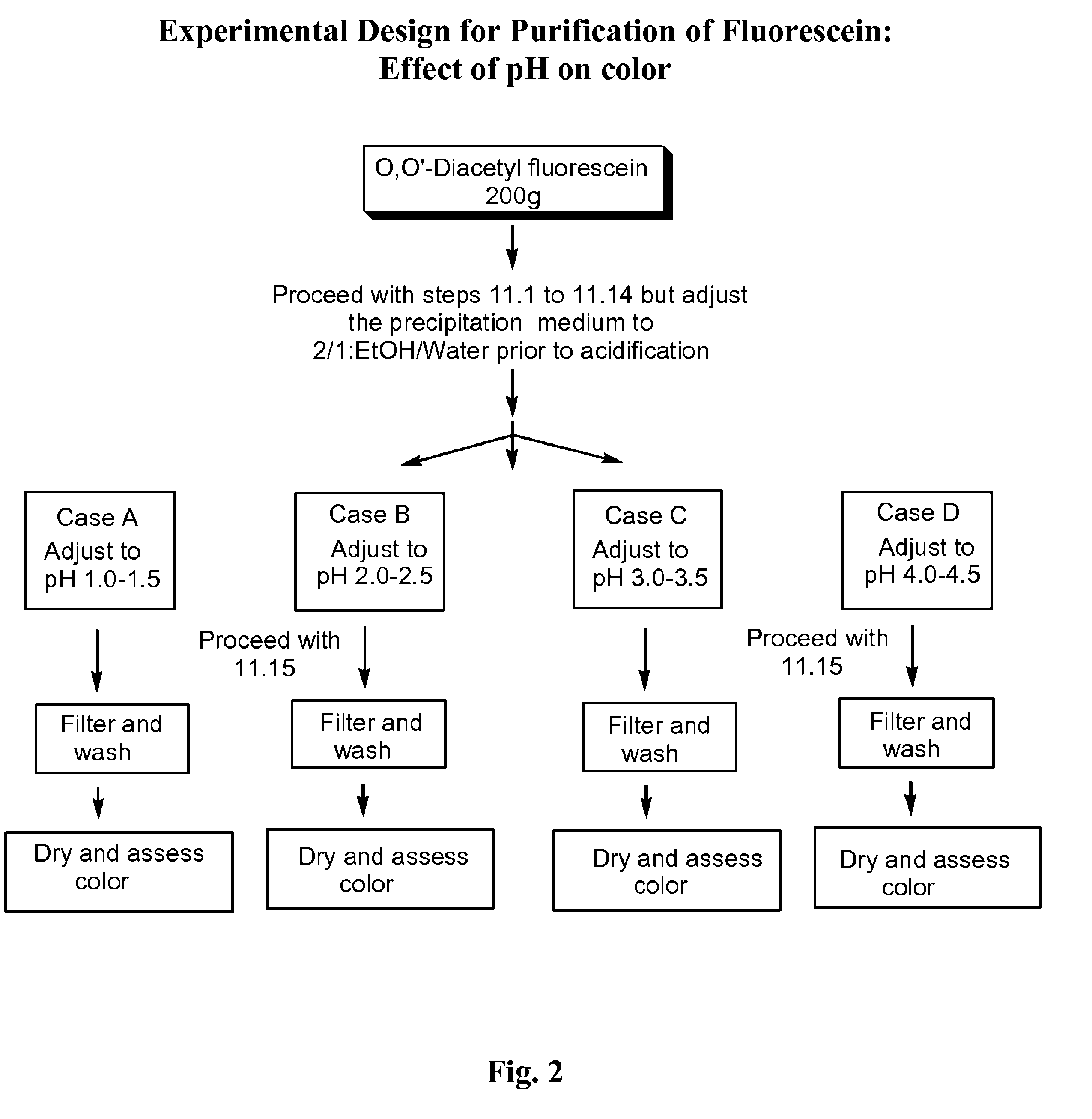
The present invention is directed to an improved process for producing substantially pure fluorescein, as well as to substantially pure fluorescein compositions prepared by the process. The invention is particularly directed to the provision of pharmaceutical compositions for use in angiography. The substantially pure fluorescein produced by the process of the present invention is low in color, low in sodium chloride content, and substantially free of pyridine.
Owner:ALCON INC
Fluorescein-based metal sensors, and methods of making and using the same
InactiveUS7160732B2High quantum yieldFeasibility of usingOrganic chemistryChemiluminescene/bioluminescencePhysical chemistryAnalytical chemistry
The present invention is directed, in part, to fluorescein-based ligands for detection of metal ions, and methods of making and using the same.
Owner:MASSACHUSETTS INST OF TECH
Detection kit for minute residues of B-cell acute lymphocyte leukemia
InactiveCN109884313AImprove the detection rateReduce missed detection rateBiological testingCD20Leukocyte Differentiation
The invention relates to the technical field of medical detection, in particular to a detection kit for minute residues of B-cell acute lymphocyte leukemia. The detection kit comprises anti CD10, CD19, CD20, CD34, CD38 and CD45 antibodies labeled by different fluoresceins. The detection kit comprises six monoclonal antibody combinations of leukocyte differentiation antigen, and a fluorescence labeling and flow cytometric detection technology is combined to realize that one-time flow cytometric detection can quickly and accurately identify the level and typing of abnormal cells of the minute residues of the B-cell acute lymphocyte leukemia, the obtained detection index can be used as an intermediate result to intuitively judge the prognosis conditions of B-cell acute lymphocyte leukemia patients, and thus important guiding significance is provided for the formulation of clinical treatment schemes for the leukemia patients.
Owner:武汉康圣达医学检验所有限公司
Immunofluorescence chromatography test strip for detecting platelet antibody and detection method of platelet antibody
ActiveCN103336122ASave resourcesSimple and fast operationMaterial analysisImmunofluorescenceFluorescein isothiocyanate
The invention discloses an immunofluorescence chromatography test strip for detecting a platelet antibody and a detection method of the platelet antibody. The immunofluorescence chromatography test strip comprises a reaction film, a sample pad, an adsorption pad and a bottom plate, wherein the reaction film is placed on the bottom plate; a detection belt and a quality control belt are fixed onto the reaction film; the sample pad is overlapped with part of one side of the reaction film and placed on the reaction film and the bottom plate; the absorption pad is overlapped with part of the other side of the reaction film and placed on the reaction film and bottom plate; a sampling point is arranged on each of two sides of the sample pad; sample and fluorescence labeled secondary antibodies are dropped and washed; and after the reaction is done, the reaction result is indicated through fluorescein isothiocyanate labeled secondary antibodies. Due to the mode, according to the immunofluorescence chromatography test strip for detecting the platelet antibody and the detection method disclosed by the invention, the detection process is simple, efficient, accurate, low in cost and practical; semi-quantitative or quantitative detection can be realized; safe, efficient and scientific platelet transfusion in clinic can be guaranteed; and the precious platelet resources also can be saved.
Owner:SUZHOU GUOKE MEDICAL TECH DEV CO LTD
Methods of use for car t cells
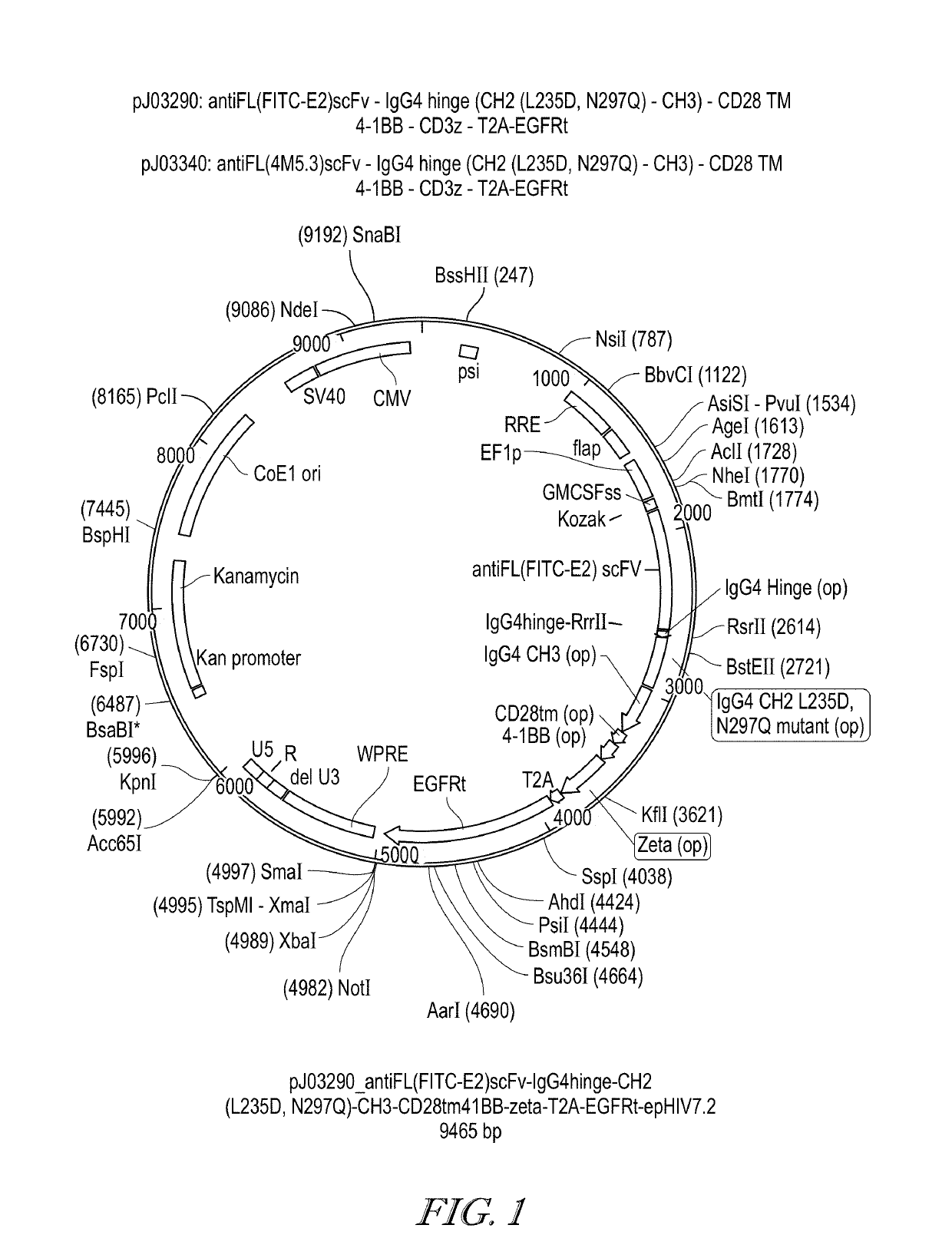
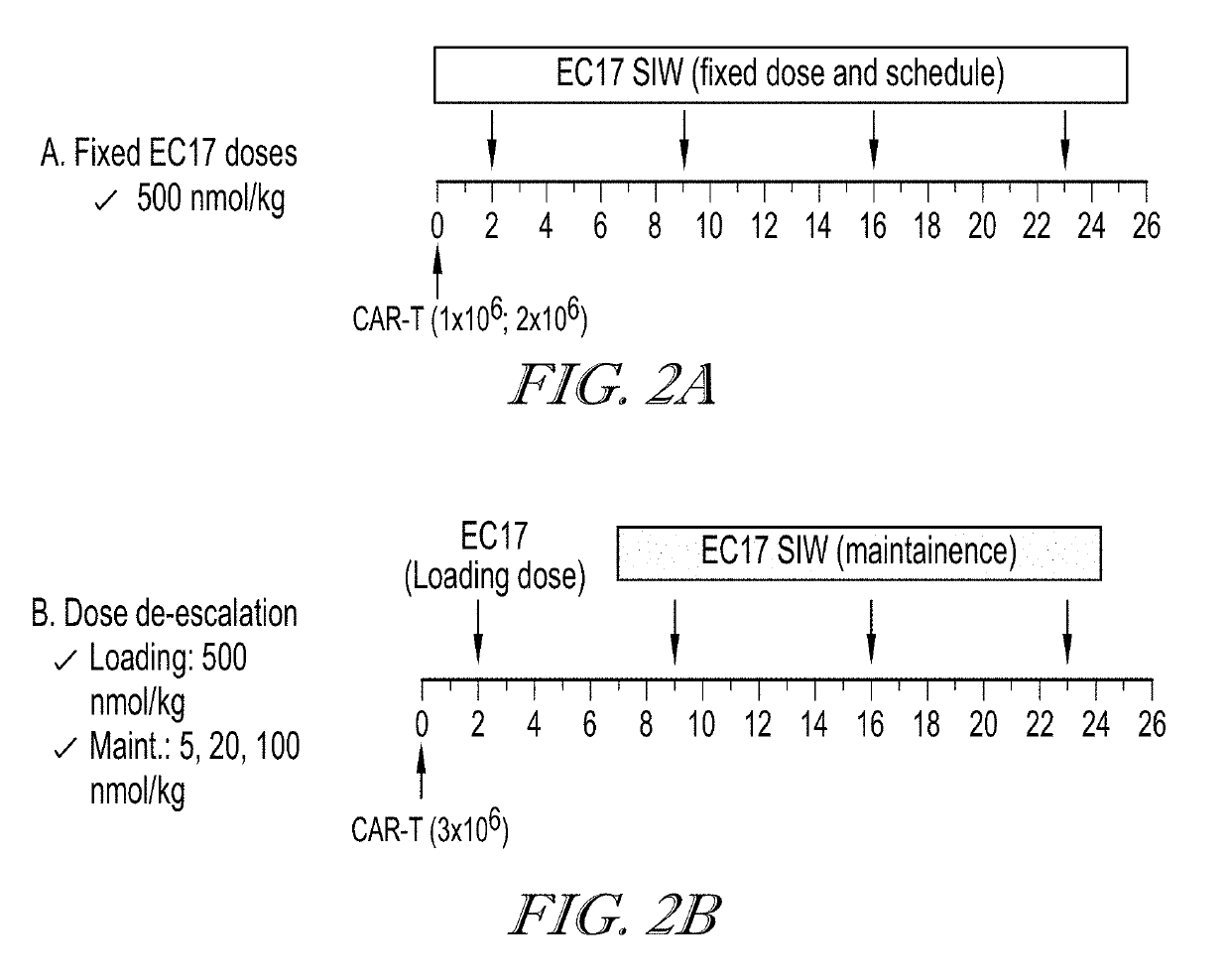
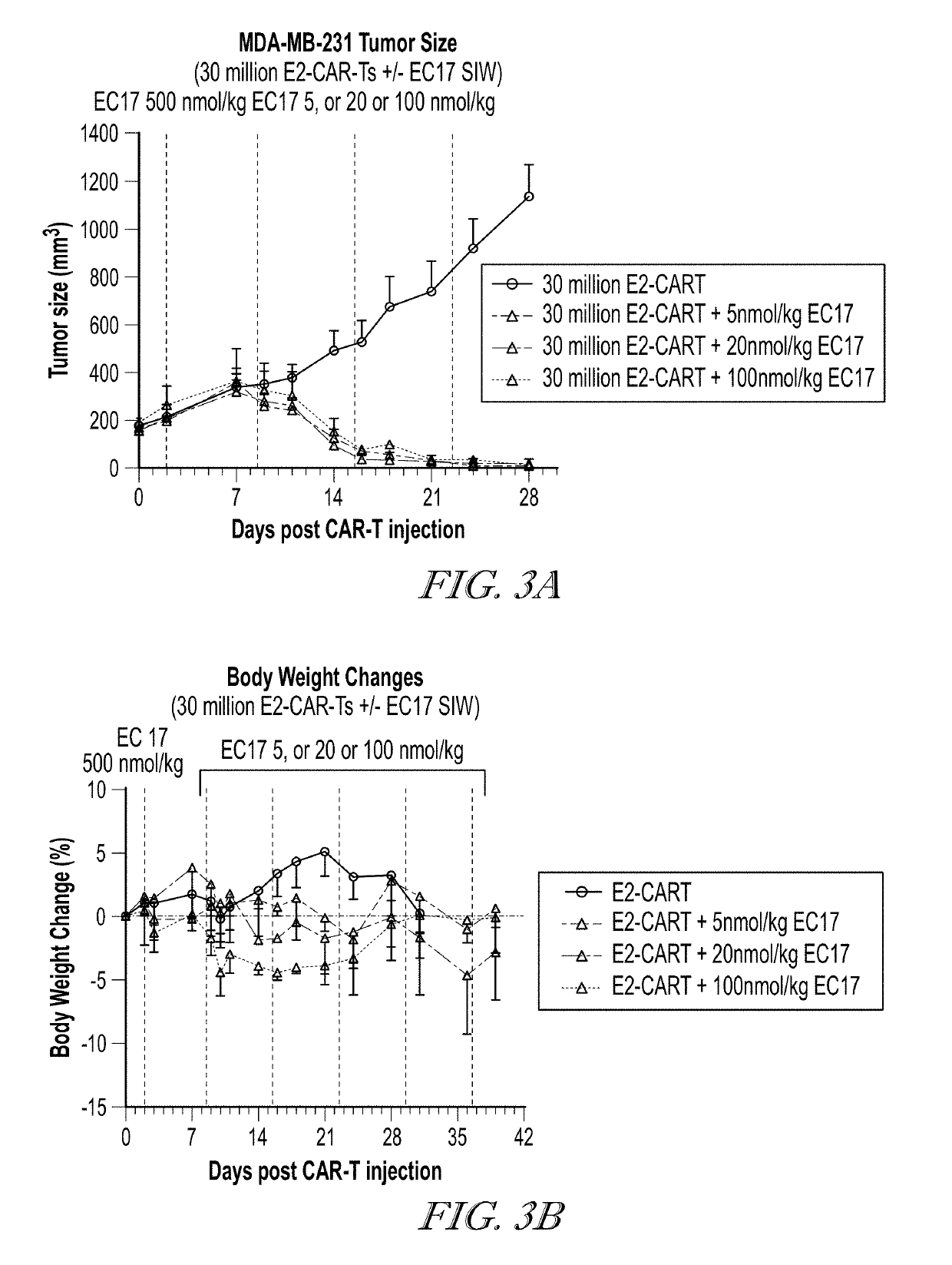
ActiveUS20190255109A1Inhibit and prevent cytokine release syndromeBody weight loss due to CRS is reduced or preventedOrganic active ingredientsPeptide/protein ingredientsAntiendomysial antibodiesAntibody fragments
The present disclosure relates to methods of treating a patient with a cancer by administering to the patient a composition comprising CAR T cells wherein the CAR T cells comprise a CAR and the CAR comprises an E2 anti-fluorescein antibody fragment, and administering to the patient a small molecule linked to a targeting moiety by a linker. The disclosure also relates to compositions for use in such methods.
Owner:PURDUE RES FOUND INC +2
Nanometer magnetic particle chemiluminescent assay kit for cancer antigen CA15-3, and preparation method and detection method thereof
InactiveCN103048461ALow costAnalysis of small differences between batchesChemiluminescene/bioluminescenceAntigenCA15-3
The invention relates to a nanometer magnetic particle chemiluminescent assay kit for a cancer antigen CA15-3, and a preparation method and a detection method thereof. The kit comprises a solution containing a fluorescein-mark labeled cancer antigen CA15-3 antibody, suspension of fluorescein antibody coated magnetic particles and a solution containing an alkaline phosphatase labeled cancer antigen CA15-3 antibody, wherein the alkaline phosphatase labeled cancer antigen CA15-3 antibody is formed by connecting alkaline phosphatase and the cancer antigen CA15-3 antibody through a crosslinking agent SMCC and 2-IT. According to the invention, the cancer antigen CA15-3 can be quantitatively detected with low cost, high accuracy degree and high precision degree.
Owner:SUZHOU HAOOUBO BIOPHARML
Compounds for detecting tyrosinase as well as preparation method and application of compounds
ActiveCN109608474AHigh chemoselectivityExcellent signal-to-noise ratioOrganic chemistryFluorescence/phosphorescenceCancer cellHydrogen
The invention belongs to the field of biochemical industry, and particularly relates to a preparation method and application of compounds FL-O-T for detecting tyrosinase. The compounds FL-O-T have a structural general formula shown in the description, wherein R1, R2 and R3 are hydrogen, alkyl or other common substituent groups. The compounds FL-O-T can be used as a fluorescent probe for detectingthe tyrosinase, and the compounds FL-O-T are synthesized by a nucleophilic substitution reaction of a fluorescein derivative and m-hydroxybenzyl bromide. The compounds FL-O-T provided by the inventionare synthesized by the nucleophilic substitution reaction of the fluorescein derivative FT-OH and the m-hydroxybenzyl bromide; and the target compounds have high chemoselectivity, can track and detect the tyrosinase in vitro and in vivo, and successfully monitor activity of the tyrosinase in cancer cells and zebrafish.
Owner:HUBEI UNIV
Nanometer magnetic particle chemiluminescence detection kit for carbohydrate antigen CA19-9 as well as preparation method thereof and detecting method thereof
ActiveCN103048453AReduce manufacturing costAnalysis of small differences between batchesChemiluminescene/bioluminescenceAntigenAntibody
The invention relates to a nanometer magnetic particle chemiluminescence detection kit for a carbohydrate antigen CA19-9 as well as a preparation method thereof and a detecting method thereof. The kit comprises a solution, which contains a fluorescein labeled carbohydrate related antigen CA19-9, a suspension, which is coated with a fluorescein antibody, and a solution, which contains an alkaline phosphatase labeled carbohydrate related antigen CA19-9 antibody, wherein the alkaline phosphatase labeled carbohydrate related antigen CA19-9 antibody is obtained by connecting an alkaline phosphatase and the carbohydrate related antigen CA19-9 antibody through cross-linking agents SMCC and 2-IT. According to the invention, the carbohydrate related antigen CA19-9 can be quantitatively detected with lower cost, higher accuracy and higher precision.
Owner:SUZHOU HAOOUBO BIOPHARML
Hard-water-resistant anti-freezing cooling liquid composition
The invention relates a hard-water-resistant anti-freezing cooling liquid composition, and provides a hard-water-resistant anti-freezing cooling liquid having the anti-boiling and anti-corrosive functions in order to overcome the conventional technical shortages. The hard-water-resistant anti-freezing cooling liquid composition is composed of glycol, sodium triazinyltriaminohexanoate, N-caprylyl sodium glutamate, a copper aluminum corrosion inhibitor, a mildew inhibitor, an antifoaming agent, fluorescein, and water.
Owner:张亚利
Confining liquid and application thereof
InactiveCN110646335AThe result is accurateGood sealingIndividual particle analysisFluorescence/phosphorescenceAntiendomysial antibodiesPolyethylene glycol
The invention relates to the technical field of confining liquid, in particular to confining liquid and application thereof. The invention discloses confining liquid. The confining liquid comprises first confining liquid and second confining liquid; the first confining liquid comprises Twain-20, donkey serum, bovine serum albumin and a phosphate buffer solution; the second confining liquid comprises Twain-20, donkey serum, bovine serum albumin, a phosphate buffer solution and polyethylene glycol octyl phenyl ether. According to the confining liquid provided by the invention, the condition thatFcR on a cell surface can be non-specifically combined with the Fc segment of a fluorescein coupling antibody effectively be reduced, and the confining effect is good, so that the result of flow cytometry is more accurate, and the confining liquid is unlikely to go bad.
Owner:GUANGDONG UNIV OF TECH
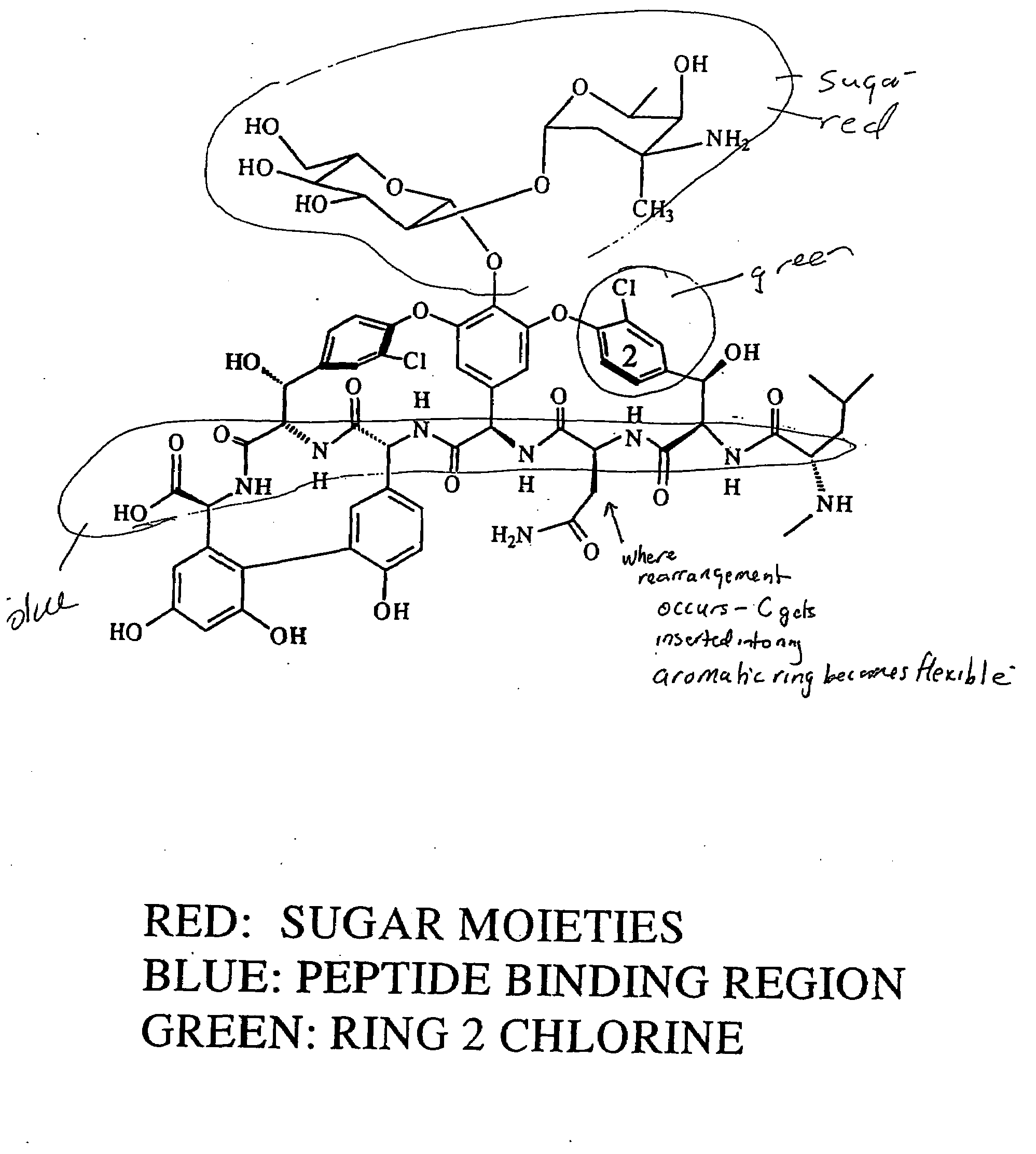
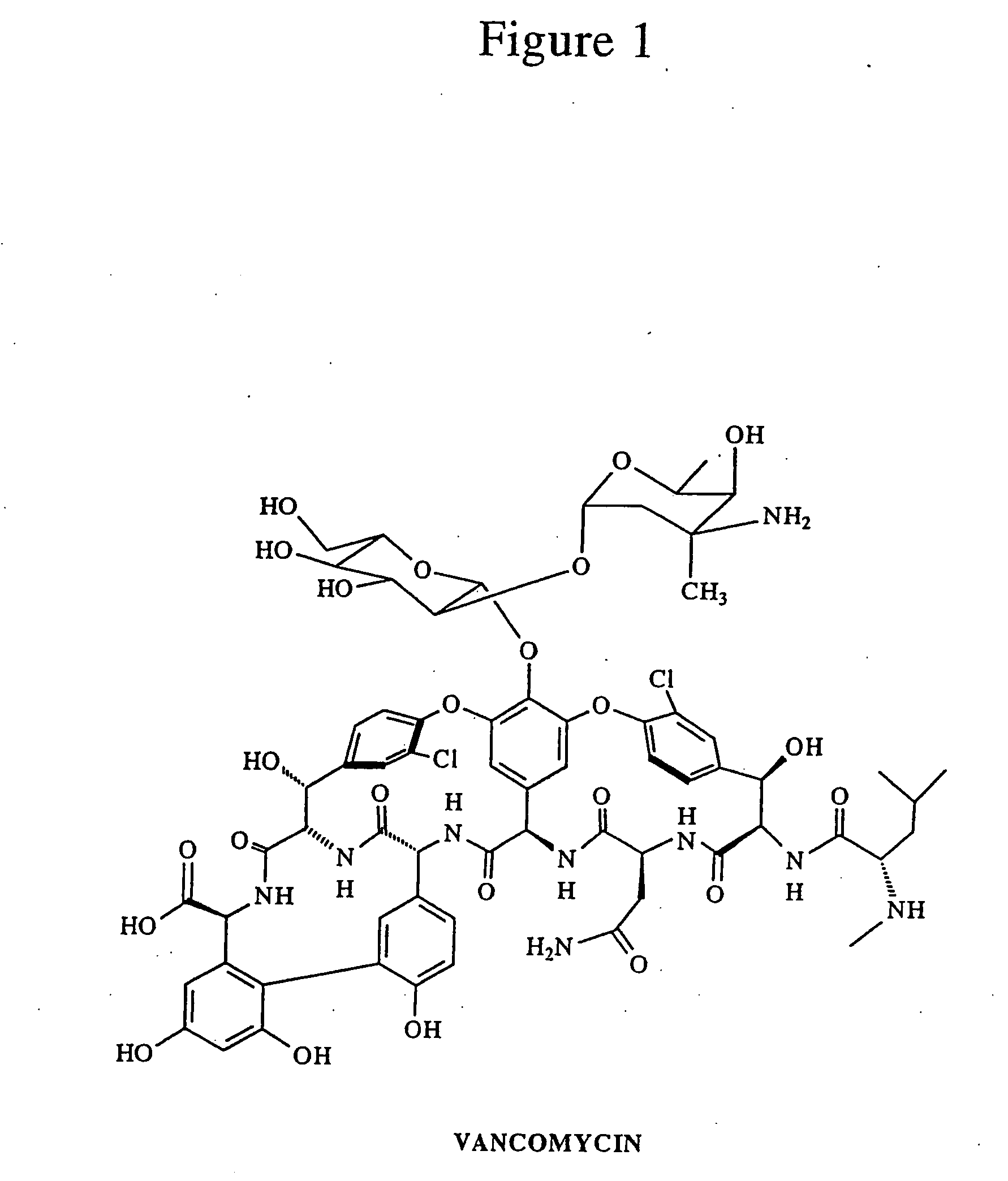
Reagents and methods for the detection and quantification of vancomycin in biological fluids
InactiveUS20080206788A1Interference be notImmunoglobulins against animals/humansHybrid cell preparationFluoresceinTest sample
Immunoassay reagents, methods and test kits for the specific quantification of vancomycin in a test sample are disclosed. The reagent comprises antibodies prepared with immunogens of FIG. 6 wherein P is an immunogenic carrier material and X is a linking moiety.Also described is the synthesis of labeled reagents of FIG. 8 wherein Q is a detectable moiety, preferably fluorescein or a fluorescein derivative, and X is a linking moiety.
Owner:ADAMCZYK MACIEJ +3
Transgenic cell with in-vivo tracking and oncotherapy functions and preparation method thereof
ActiveCN103695373AEvaluate efficacyTumor targetingFermentationAntineoplastic agentsRenilla luciferaseWilms' tumor
The invention discloses a transgenic cell with in-vivo tracking and oncotherapy functions and a preparation method thereof. The transgenic cell is a mesenchymal stem cell from a human umbilical cord, is capable of performing oncotherapy, has a molecular imaging function, and is capable of monitoring the body distribution and survival condition in real time in a living body state after being subjected to intratumor injection. Particularly, a renilla luciferase-red fluorescence protein-suicide gene thymidine kinase fusion gene is transfected in the mesenchymal stem cell from the human umbilical cord, and a fluorescein signal is collected by means of a living body imaging system to monitor targeted therapy of a Nude mouse breast cancer model; a mouse injected with the mesenchymal stem cell transfected with the fusion gene is administrated with a substrate ganciclovir of thymidine kinase, and the death of tumor cells around the mesenchymal stem cell is induced through the bystander effect.
Owner:NANKAI UNIV +1
Method for detecting concentration of trace immunoglobulin G in human serum
InactiveCN103969446AAccurate, convenient and fast detectionHigh sensitivityBiological testingFluorescence/phosphorescenceReceptorIntravenous gammaglobulin
The invention discloses a method for detecting the concentration of trace immunoglobulin G in human serum. An energy transferring system with stable performance consists of goat anti human immunoglobulin G antibody-labeled fluorescein isothiocyanate serving as an energy transferring supplier and nano gold serving as a receptor; the goat anti human immunoglobulin G antibody-labeled fluorescein isothiocyanate transmits energy to the nano gold, so that fluorescence of the fluorescein isothiocyanate is quenched; however, after immunoglobulin G is added, the fluorescence of the fluorescein isothiocyanate is recovered; furthermore, the fluorescence recovery value and the concentration of the immunoglobulin G are in good linear relation within a range of 4.0-220.0 nanograms per milliliter, so that a novel method for detecting the immunoglobulin G is built. According to the method disclosed by the invention, the shortcomings of low sensitivity, complicated operation and the like during detection in the prior art are overcome, and the immunoglobulin G in the human serum can be conveniently and quickly detected.
Owner:GUILIN UNIVERSITY OF TECHNOLOGY
Novel colored solutions of injectable drugs and their pharmaceutically acceptable salts
InactiveCN101400369AUltrasonic/sonic/infrasonic diagnosticsInfrasonic diagnosticsAnticholinergic agentsEmulsion
The invention is directed to pharmaceutical compositions comprising colored solutions, colored emulsions, or colored powders of injectable pharmaceuticals wherein said pharmaceuticals are selected from the group consisting of muscle relaxants, hypnotics, induction agents, and anticholinergics. The formulations of the present invention may all be colored using fluorescein. Different colors may be achieved by either varying the concentration of fluorescein, or by combining fluorescein with another dye. The invention is also directed to methods involving the use of said pharmaceutical compositions.
Owner:P·D·温施
Porcine deltacoronavirus LFD-RPA rapid detection primer probe set and kit
InactiveCN110241265AQuick checkStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationNucleotideFluorescein
The invention relates to a porcine deltacoronavirus LFD-RPA rapid detection primer probe set and a kit, and belongs to the field of biotechnology. The primer probe set includes an upstream primer, a downstream primer and a probe; the nucleotide sequence of the upstream primer is shown in SEQ ID NO.1, the downstream primer is a substance of which the nucleotide sequence is shown in SEQ ID NO.2 and the 5' end is modified by biotin, and the probe is a substance of which the nucleotide sequence is shown in SEQ ID NO.3, the 5' end is modified by C3Spacer, the 5' end is modified with fluorescein and the thirtieth base from the 5' end is modified by a tetrahydrofuran residue. By adopting the porcine deltacoronavirus LFD-RPA rapid detection primer probe set, porcine deltacoronaviruses can be rapidly detected; the primer probe set is simple and high in sensitivity and specificity and can improve the effectiveness of a current PDCoV controlling scheme.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
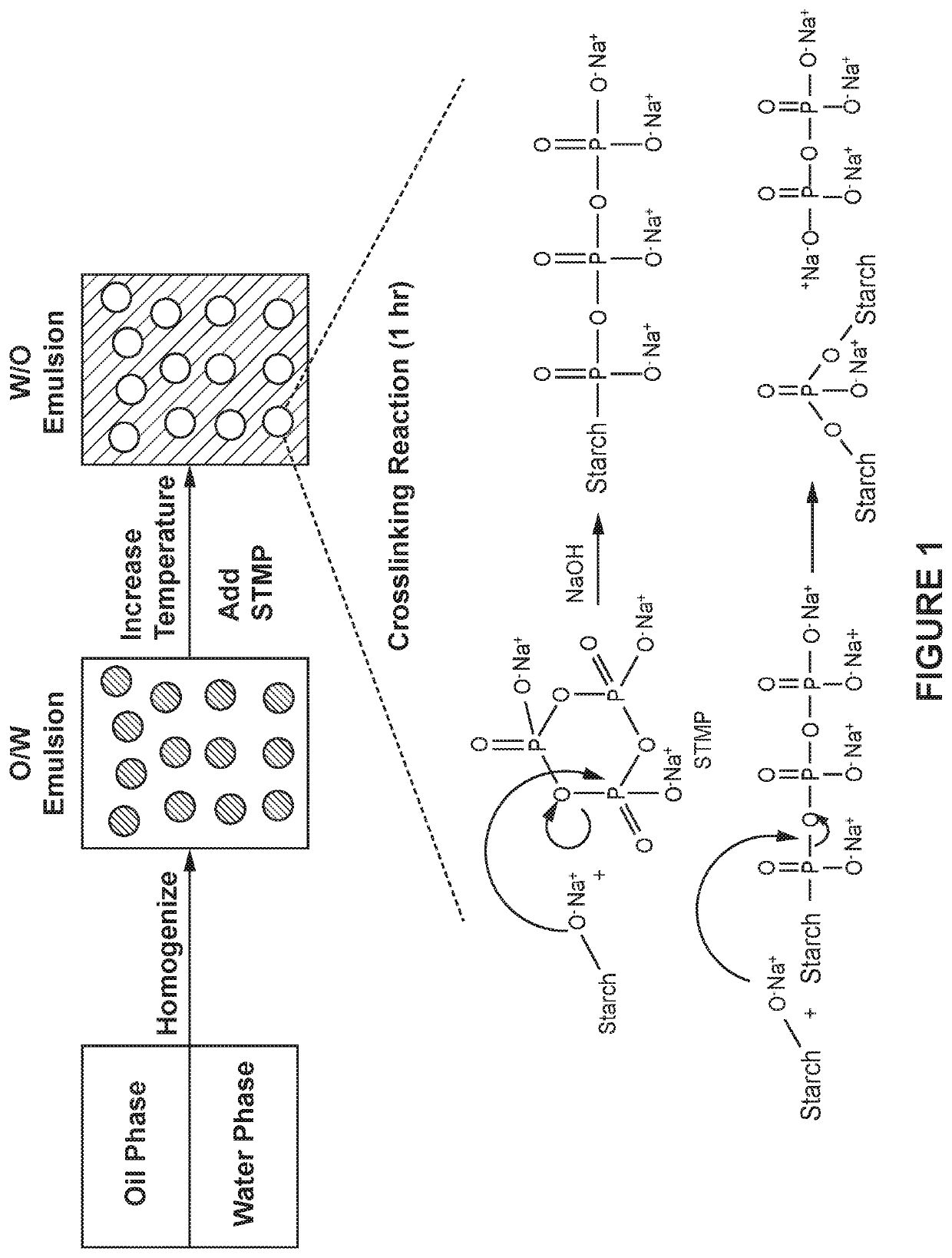
Phosphate crosslinked starch nanoparticle and dental treatments
ActiveUS20210000710A1Cosmetic preparationsOrganic active ingredientsActive agentTooth Remineralization
A phosphorous compound such as STMP is used as a cross-linking agent while making a starch nanoparticle in an emulsion process. Negative charge of the nanoparticle is reduced or reversed by adding cations and / or cationizing the starch optionally while forming the nanoparticles. Anionic active agents, such as fluoride or fluorescein, are optionally incorporated into the nanoparticle during the formation process. For example, a fluoride salt can also be used, which promotes the crosslinking reaction while also providing fluoride in the nanoparticle. The retention of both calcium and fluoride in the nanoparticle is improved when both salts are used. Alternatively, the nanoparticle may be used without added calcium and / or fluoride. The nanoparticles may be useful for tooth remineralization, the treatment of dentinal hypersensitivity, to treat caries, or as a diagnostic agent to locate carious lesions.
Owner:GREENMARK BIOMEDICAL INC
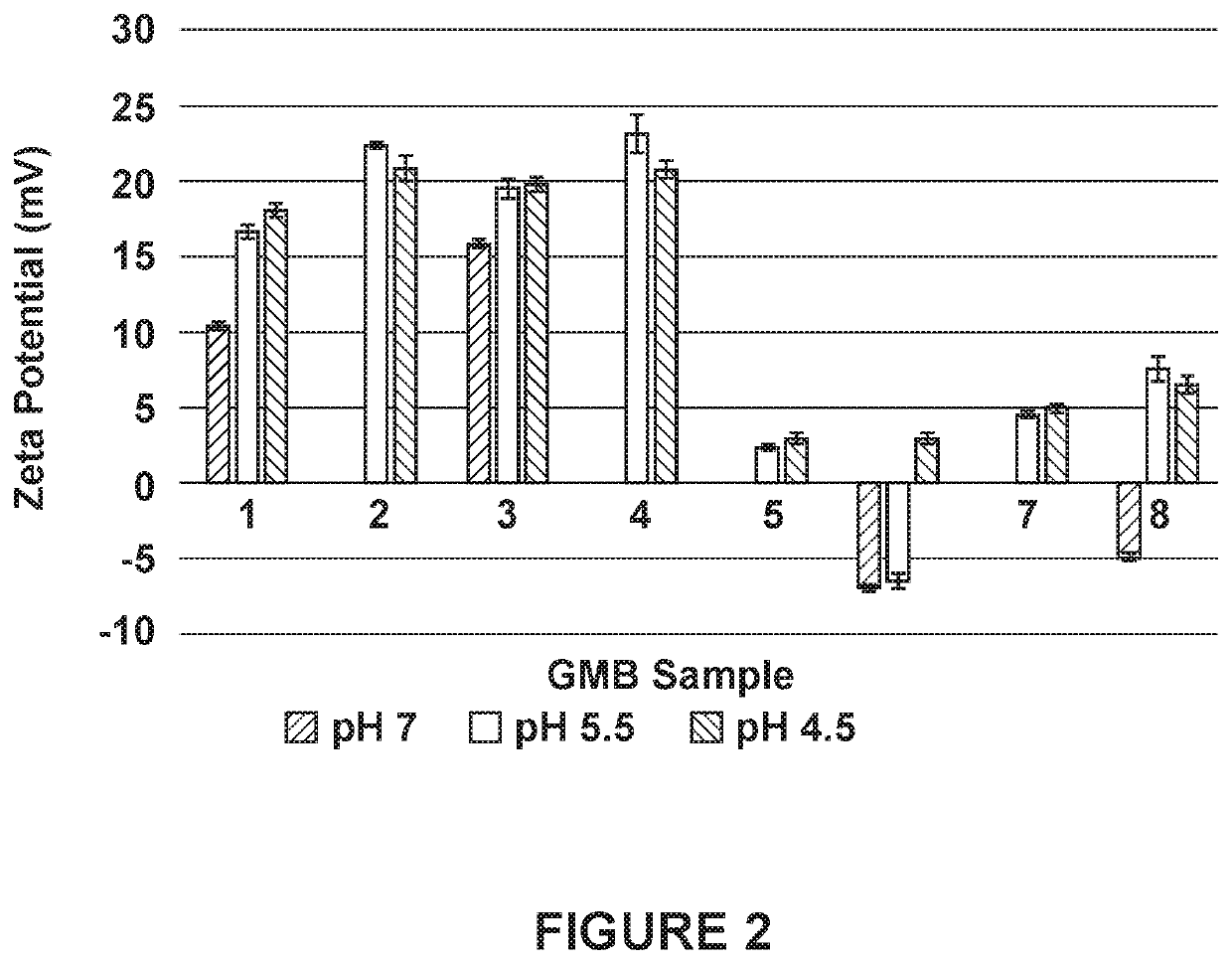
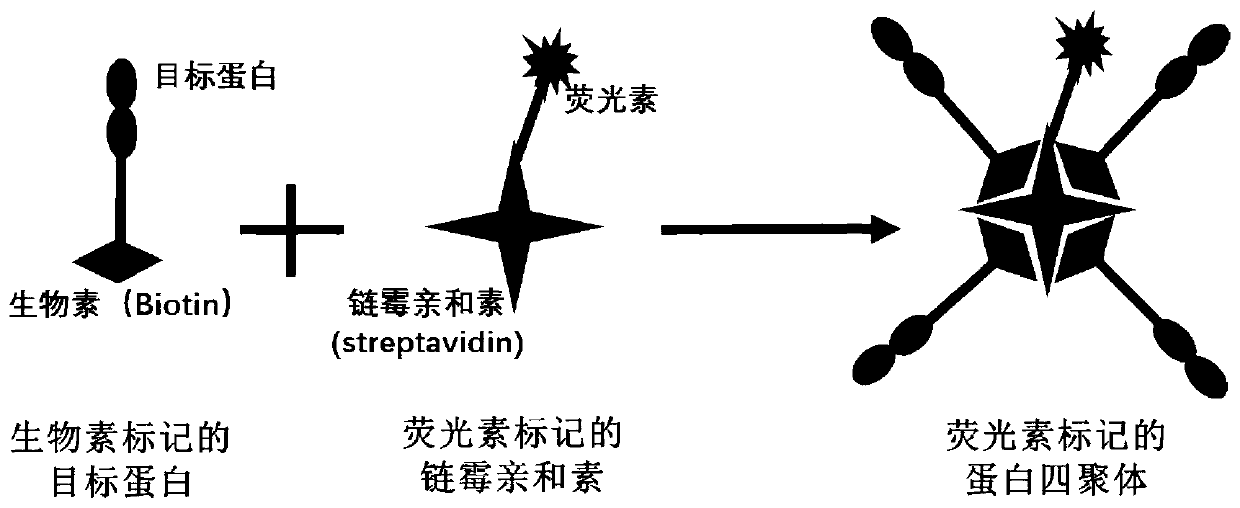
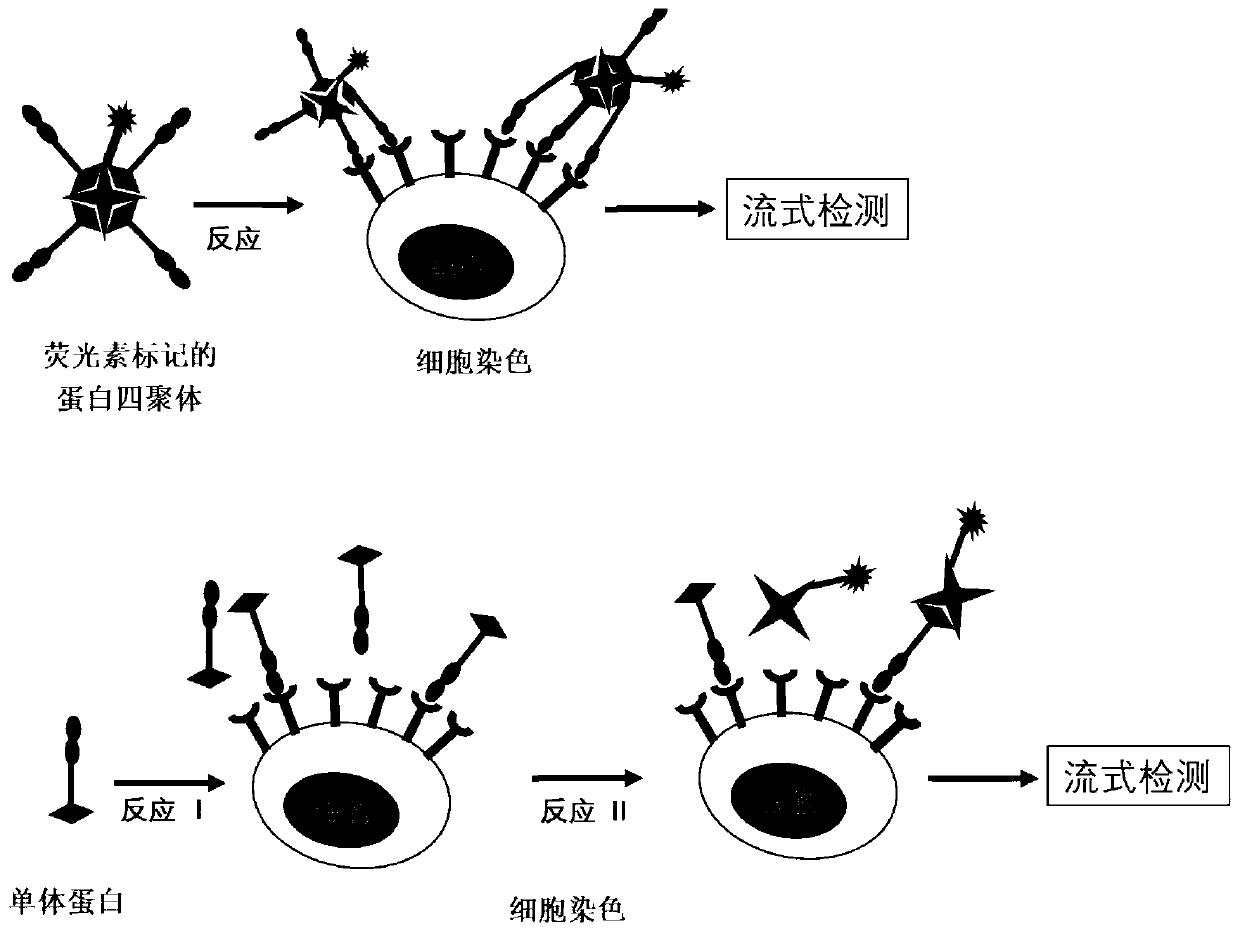
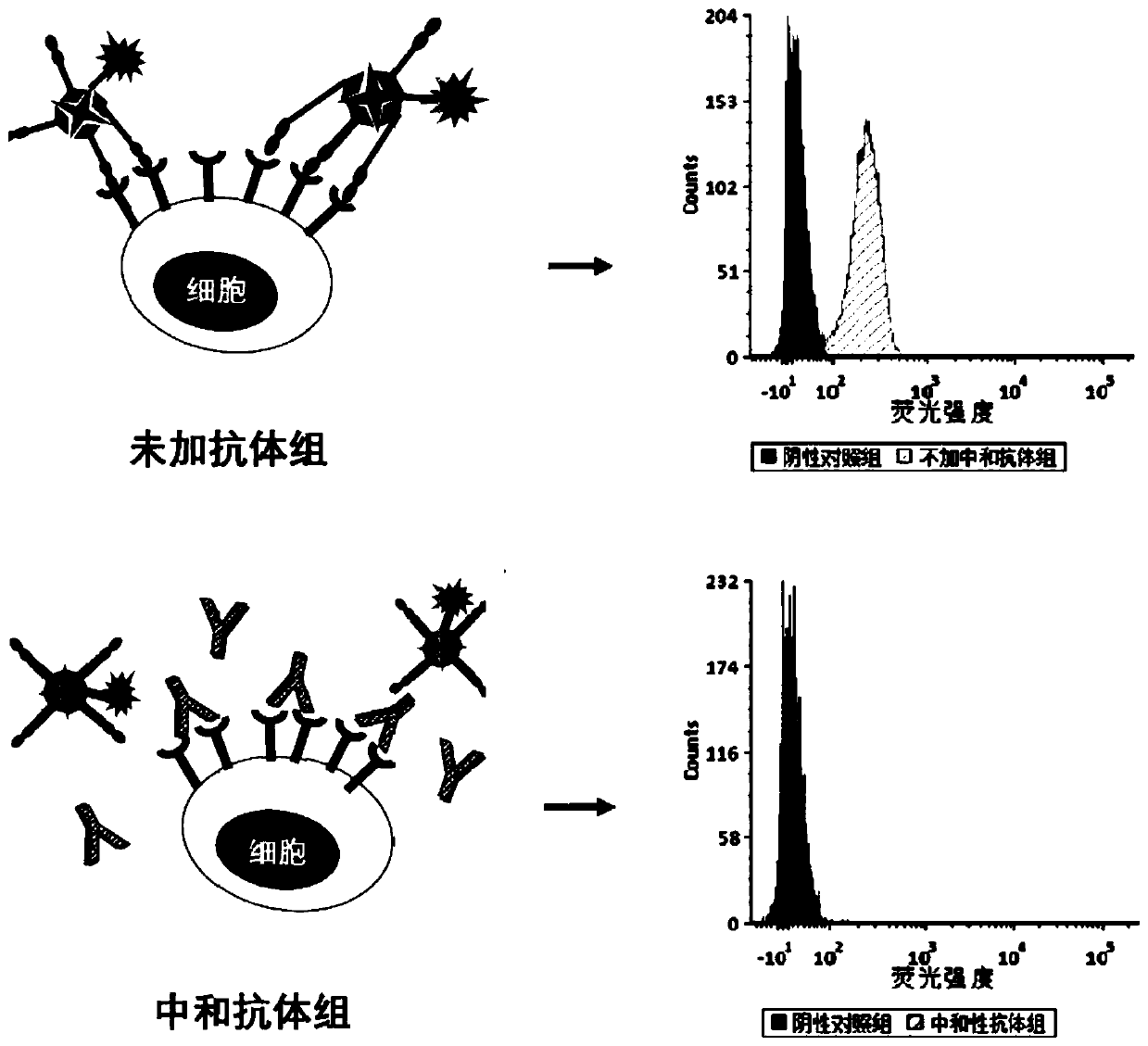
Fluorescein marked protein tetramer as well as preparation method and application thereof
ActiveCN111100199AHigh detection sensitivityCell receptors/surface-antigens/surface-determinantsBiological material analysisAntigenProtein target
The invention provides a fluorescein marked protein tetramer as well as a preparation method and application thereof. The fluorescein marked protein tetramer is formed by combining fluorescein markedstreptavidin and a biotin marked target protein. The biotin marked target protein is in recombinant expression in an HEK293 cell, one Avi Tag is coexpressed at an N-end or a C-end, and furthermore, under the action of a BirA enzyme, a lysine residue group of Avi Tag is connected with biotin. The biotin marked target protein and the fluorescein marked streptavidin are mixed and reacted to obtain the protein tetramer in direct fluorescence marking. The protein tetramer in direct fluorescence marking, which is provided by the invention, can be applied to detection on positive rates of neutralizing antibodies or CAR-T (chimeric antigen recetor-T) cells of target proteins of stream screening immune checkpoints, and as streptavidin and biotin both play a role of cascade amplification, the detection sensitivity of the protein tetramer is greatly improved when being compared with that of fluorescein marked proteins prepared by using other methods.
Owner:ACROBIOSYSTEMS INC
Fluorescence method and kit for testing lymphocyte homing receptor
InactiveCN103674913ASimple and fast operationQuick responseFluorescence/phosphorescenceCD44Lymphocyte
The invention discloses a fluorescence method a kit for testing a lymphocyte homing receptor, the fluorescence method is as follows: a cationic conjugated polymer is bonded with fluorescein-marked negatively-charged hyaluronic acid by electrostatic attraction for fluorescence resonance energy transfer from the conjugated polymer to the fluorescein, and the fluorescence intensity of the fluorescein is increased significantly; when CD44 ( lymphocyte homing receptor) is added, specific bonding of the CD44 and the hyaluronic acid is happened, the bonding of the cationic conjugated polymer and the hyaluronic acid with fluorescein groups is weakened, the distance between molecules is increased, so that the energy transfer efficiency is reduced, the conjugated polymer fluorescence is gradually restored, the fluorescein fluorescence is gradually weakened, and the energy transfer efficiency reducing degree is relative to the concentration of the CD44. The method is simple in operation, fast in response speed, low in cost and high in sensitivity and selectivity, and has important significances in biological detection and early diagnosis and treatment of diseases.
Owner:深圳云安智慧医疗科技有限公司
Flow type staining kit and preparation method and application method thereof
The invention discloses a flow type staining kit and a preparation method and an application method thereof, and particularly relates to a preparation method and an application method of a flow type staining kit for detecting various protein molecules in mammal nucleated cell cytoplasm, and the kit comprises a flow type intracellular fixation / membrane rupture solution and a flow type intracellular membrane rupture / washing solution. The immobilization / membrane rupture liquid is composed of paraformaldehyde, saponin and a phosphate buffer solution; the membrane rupture / washing liquid consists of saponin, fetal calf serum, a metal ion chelating agent, a phosphate buffer solution and a preservative. The kit can be used in combination with various fluorescein-labeled antibodies, and cell membranes are perforated through membrane rupture / washing liquid, so that fluorescence-labeled antibody molecules enter cytoplasm to be combined with specific antigen proteins. And the cells are detected through a flow cytometer to obtain information of protein molecule expression in specific cytoplasm. The invention can be used for flow immunodetection of various cells.
Owner:苏州东岭生物技术有限公司
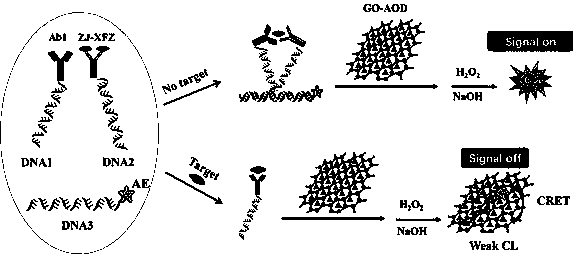
Homogeneous immunoassay method for quenching acridinium ester chemiluminiscence based on ortho-position touch effect and graphene oxide and use equipment
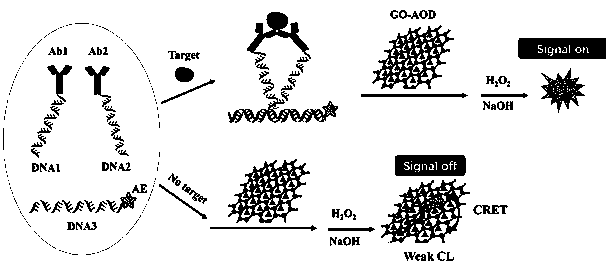
ActiveCN111007239AReduce turnaround timeEasy to operateChemiluminescene/bioluminescenceBiological testingImmune profilingAntiendomysial antibodies
The invention relates to the technical field of chemiluminiscence immunoassay, in particular to a homogeneous immunoassay method for quenching acridinium ester chemiluminiscence based on an ortho-position touch effect and acridinium ester chemiluminiscence and use equipment. A detection solution adopted in the analysis method comprises a DNA1-conjugate, a DNA2-conjugate, acridinium ester (AE) labeled DNA3, a graphene oxide (GO) combined antioxidant (AOD) and the like. The method is a homogeneous chemiluminiscence immunoassay method, separation and cleaning steps are not needed, the operation is simple, meanwhile, the turnover time (TAT) of clinical examination specimens is greatly shortened, centrifugal treatment is not needed for blood specimens, whole blood loading can be achieved, and adetection report can be given within 5-10 minutes; the acridinium ester derivative (AE) is different from fluorescein (Cy5 and the like) substances and can realize anti-interference chemiluminescencedetection; and the method not only can be used for detecting macromolecular protein and antibodies, but also can be used for realizing immunodetection of chemical small molecules.
Owner:南京浦光生物科技有限公司
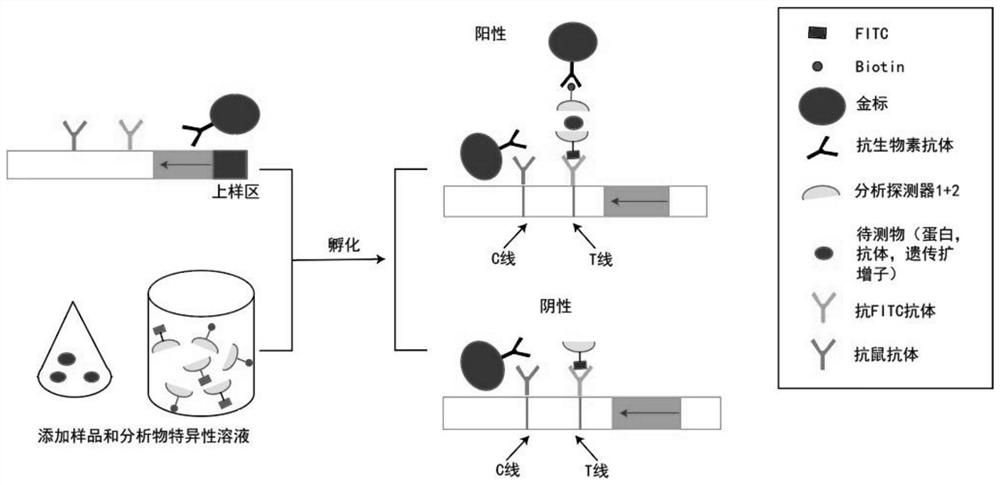
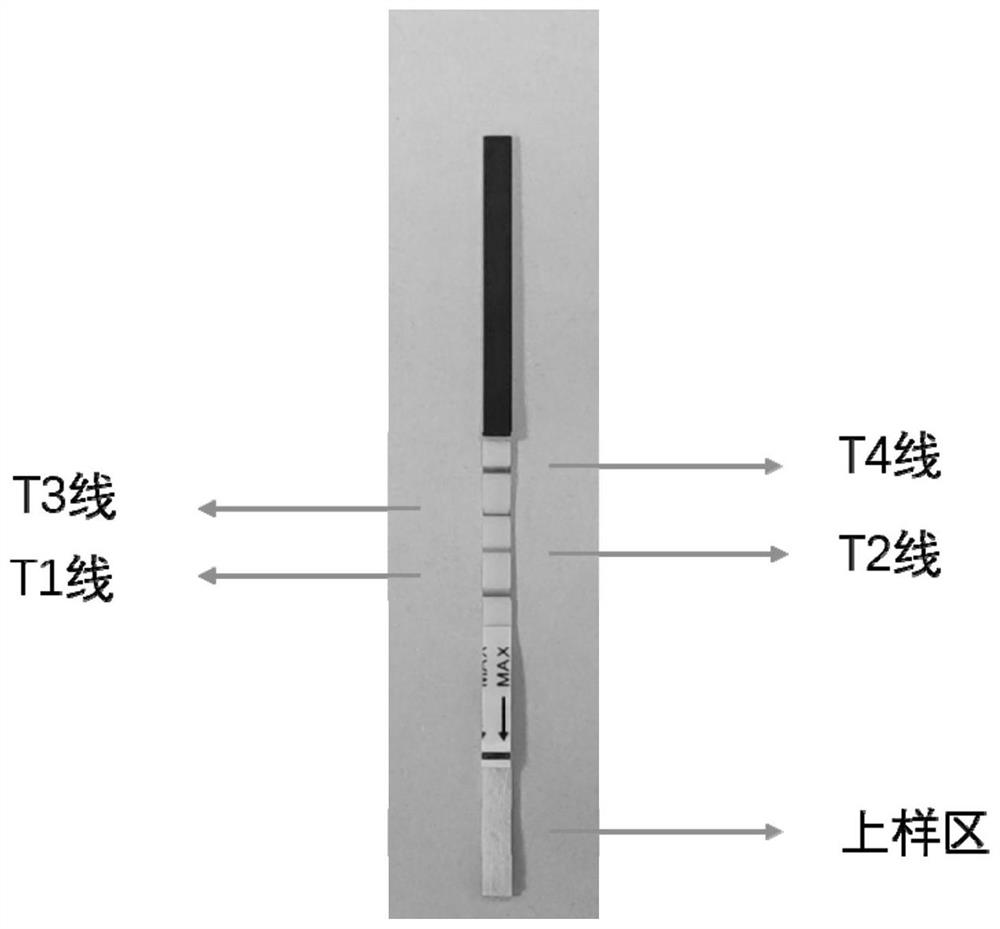
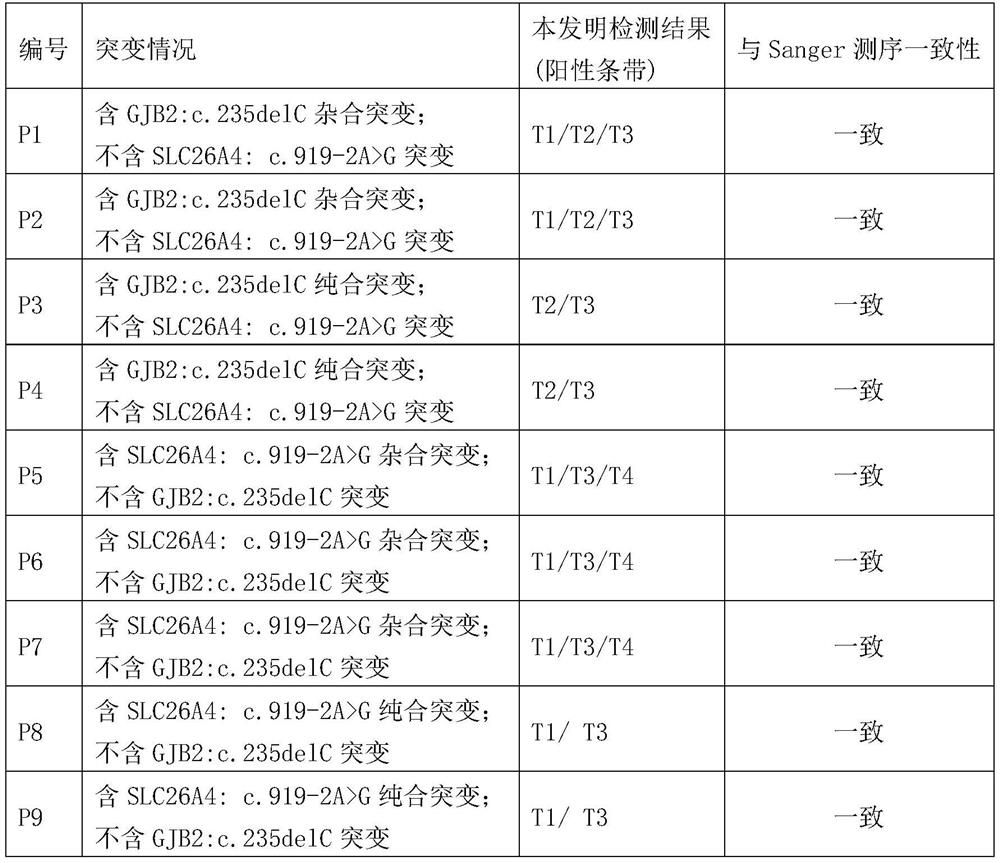
Rapid, simple and convenient deafness gene detection method and kit
InactiveCN113106152ALower quality requirementsLower requirementMicrobiological testing/measurementMaterial analysisBiotinGene Mutant
The invention discloses a rapid, simple and convenient deafness gene detection method and a kit. The kit comprises a primer combination for detecting deafness gene mutation, the primer combination is composed of G235WF, G235MF, G235WR, SIV72WF, SIV72WR and SIV72MR, and the sequences of the primer combination are as follows: the 5'tail ends of the G235WF, the G235MF, the G235WR, the SIV72WF, the SIV72WR and the SIV72MR are respectively marked with fluorescein FITC, TAMATA, biotin biotin, biotin biotin, fluorescein FAM and DIG. Experiments prove that the kit disclosed by the invention can be used for rapidly, accurately and sensitively detecting mutation conditions of two sites of GJB2: c.235delC and SLC26A4:c.919-2A>G.
Owner:北京尔惠基因科技有限公司
Triple immunofluorescence quantitative detection kit for cardiac troponin I, brain natriuretic peptide and D-dimer chest pain
PendingCN112326975AWide detection rangeLow detection limitDisease diagnosisBiological testingI antibodyReagent strip
The invention provides a triple immunofluorescence quantitative detection kit for cardiac troponin I, brain natriuretic peptide and D-dimer chest pain. The kit for joint quantitative detection of cardiac troponin I, brain natriuretic peptide and D-dimer comprises a reagent strip, and the reagent strip comprises a substrate, and a sample pad, a CP pad, an NC membrane and an absorption pad which aresequentially pasted on the substrate; the CP pad is coated with a cardiac troponin I antibody, a brain natriuretic peptide antibody, a D-dimer antibody and a fluorescein labeled conjugate of chickenIgY; and the NC membrane is respectively provided with a detection line coated with a D-dimer monoclonal antibody, a cTnI monoclonal antibody, a BNP monoclonal antibody and a rabbit anti-chicken IgY monoclonal antibody. According to the detection kit disclosed by the invention, cTnI, BNP and D-dimer can be simultaneously, quickly and accurately detected quantitatively by one-time sampling, so thatclinical diagnosis and treatment on a patient suffering from chest pain are effectively assisted.
Owner:RELIA BIOTECH JIANGSU
ERG gene detection probe, preparation method thereof and reagent kit
InactiveCN105420398AGood repeatabilityIncrease signal brightnessMicrobiological testing/measurementDNA/RNA fragmentationErgMutation detection
The invention relates to an ERG gene detection probe and a preparation method thereof. The method comprises the following steps that two sets of BAC clones are selected, wherein one set is at least one of RP11-720N21 and CTD-2556C4, and the other set is at least one of RP11-360N24 and RP11-110N12; plasmids are extracted from the clones to obtain plasmid DNA, and quantitation is performed; fluorescein is used for labeling. The invention further discloses a reagent kit containing the ERG gene detection probe. The optimal ERG detection probe is obtained through screening, the signal counting row is accurate is rapid, and the result repeatability is good; the defect of clinical ERG mutation detection is overcome, more patients benefiting from targeted drugs are screened out, the patient survival rate and overall survival are improved.
Owner:GUANGZHOU LBP MEDICINE SCI & TECH
EBER probe for detecting EBV infected tissue and detection kit
PendingCN113151588AGood hybrid specificityThe test result is accurateMicrobiological testing/measurementMicroorganism based processesEBV InfectionsNucleotide
The invention provides an EBER probe for detecting EBV infected tissues and a detection kit, and belongs to the technical field of virus detection. The EBER probe is a fluorescein-labeled EBER probe, and the nucleotide sequence of the EBER probe is shown in SEQ ID NO: 1. The probe hybridization solution is an aqueous solution containing 10-20 pmol / [mu]l of an EBER probe, 50% of formamide, 10% of dextran sulfate, 1.0% of Triton X-100 and 50 mmol / L of Tris-HCl. The invention also provides the detection kit which comprises the probe hybridization solution, a DAPI counterstaining agent and a quality control sheet group, can realize the detection of the EBER state in a tissue or cell sample, and assists in clinically judging whether EBV infection exists or not. Through verification, the kit is high in detection sensitivity and good in specificity, the detection method is simple and convenient, the defects of an existing product or method are overcome, and the kit has wide application prospects.
Owner:GUANGZHOU LBP MEDICINE SCI & TECH
Preparation method of Cu nanometer material and cancer cell detection method
InactiveCN105478754AEasy to prepareLow costTransportation and packagingMetal-working apparatusBiocompatibility TestingPatient need
The invention discloses a preparation method of a Cu nanometer material. The preparation method comprises the following steps that bar-shaped ZnO is prepared through a direct deposition method, then ZnO is plated with Ni, nickel-plated ZnO is wrapped by Cu, and finally the Cu-wrapped nickel-plated ZnO nanometer material is modified through hyaluronic acid. A cancer cell detection method comprises the following steps that the Cu nanometer material is subjected to fluorescein marking, then the Cu nanometer material and cancer cells are cultivated together, and then put under a laser microscope to be observed, and the number of the cancer cells is quantitatively detected according to the staining quantity. The Cu nanometer material prepared through the method has the beneficial effects of being easy to prepare, good in biocompatibility, capable of being combined with the cancer cell specific receptor CD44 and the like. According to the method for utilizing the Cu nanometer materials for detecting the cancer cells, only the blood of a patient needs to be collected for in-vitro detection, the cancer cells can be recognized in few samples, and the cancer can be accurately, sensitively and atraumatically diagnosed in the initial stage of illness.
Owner:AFFILIATED YONGCHUAN HOSPITAL OF CHONGQING MEDICAL UNIV
Bioluminescent engineered bacterium composition, and preparation method and application thereof
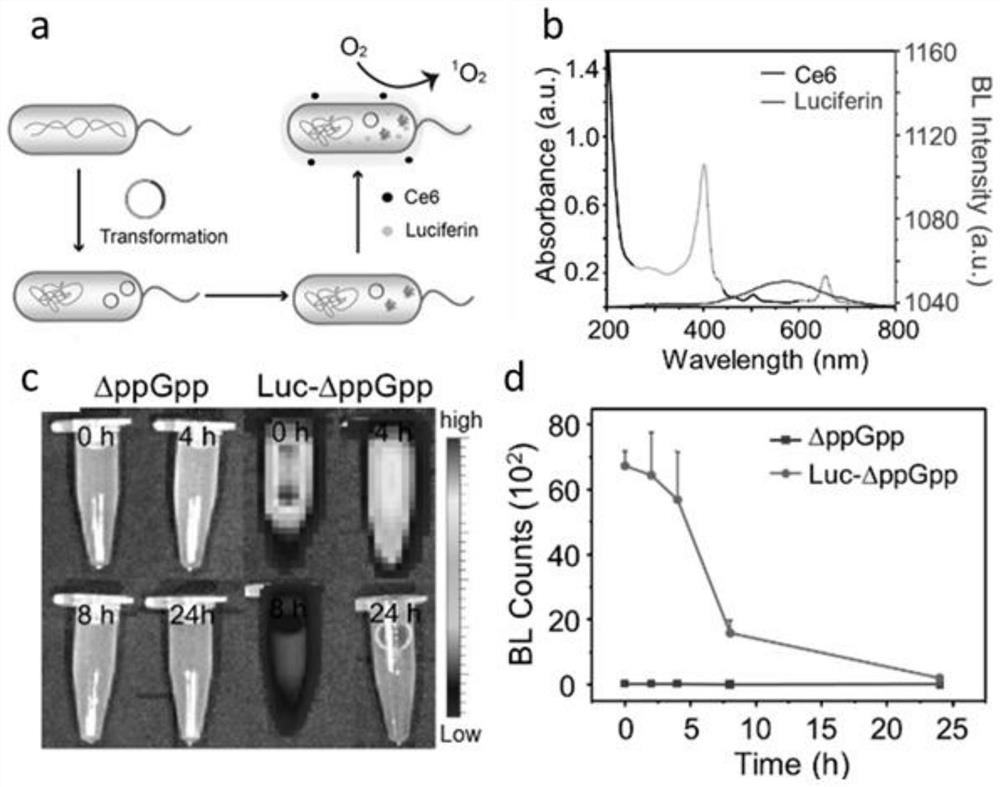
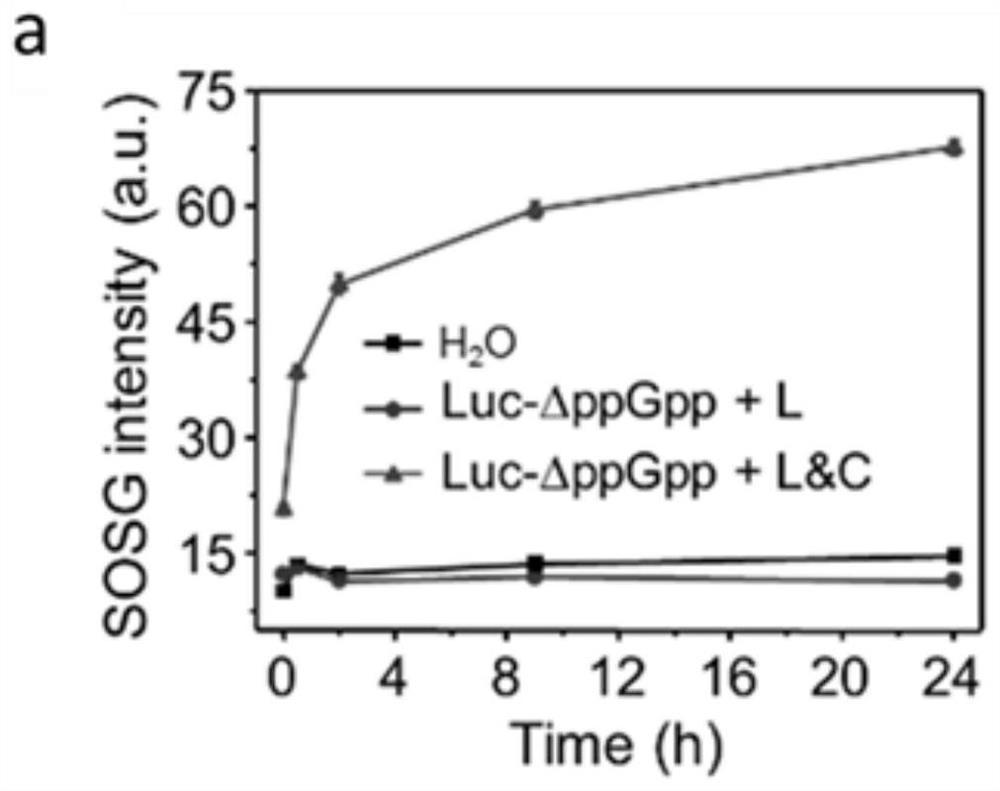
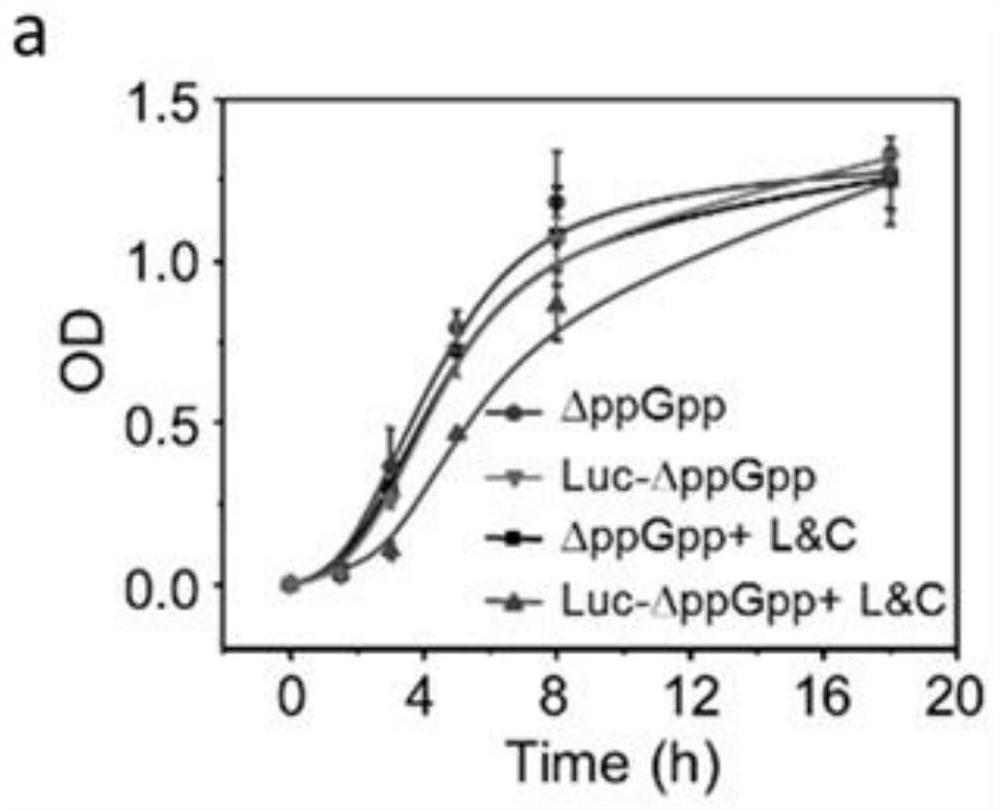
ActiveCN112546226ASolve the problem of insufficient penetration depthAddressing suboptimal monotherapyBacteriaPhotodynamic therapyGreen-lightPlasmid transfection
Owner:SUZHOU UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com