Detection kit for minute residues of B-cell acute lymphocyte leukemia
A B lymphocyte and minimal residual technology, applied in the field of medical testing, can solve the problems of poor accuracy of acute B lymphocytic leukemia minimal residual, single judgment standard, and high missed detection rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Example 1 Preparation of Minimal Residue Detection Kit for Acute B Lymphoblastic Leukemia
[0058] 1. Antibody composition: the composition of the antibody composition is as follows: CD10-PE, CD19-APC, CD20-APC-cy7, CD34-PerCP, CD38-V450, CD45-V500. That is, it consists of monoclonal antibodies against CD10, CD19, CD20, CD34, CD38 and CD45 labeled with PE, APC, APC-cy7, PerCP, V450 and V500 fluorescein in sequence.
[0059] 2. Red blood cell lysate: purchased from BD Company, including the following components: ammonium chloride, potassium dihydrogen phosphate, disodium edetate, and paraformaldehyde.
[0060] 3. Antibody dilution reagent: The composition of the antibody dilution reagent is as follows:
[0061] Fetal bovine serum 1.5% (mass volume percentage content);
[0062] Phosphate buffer 0.01M;
[0063] The pH is 7.2-7.4.
[0064] The preparation method of the antibody dilution reagent is as follows: 0.1g-0.2g of fetal bovine serum is weighed and dissolved in 10m...
Embodiment 2
[0066] Example 2 Detection method for minimal residues of acute B-lymphoblastic leukemia
[0067] This embodiment provides the detection method of the detection kit prepared in the above embodiment 1, specifically as follows:
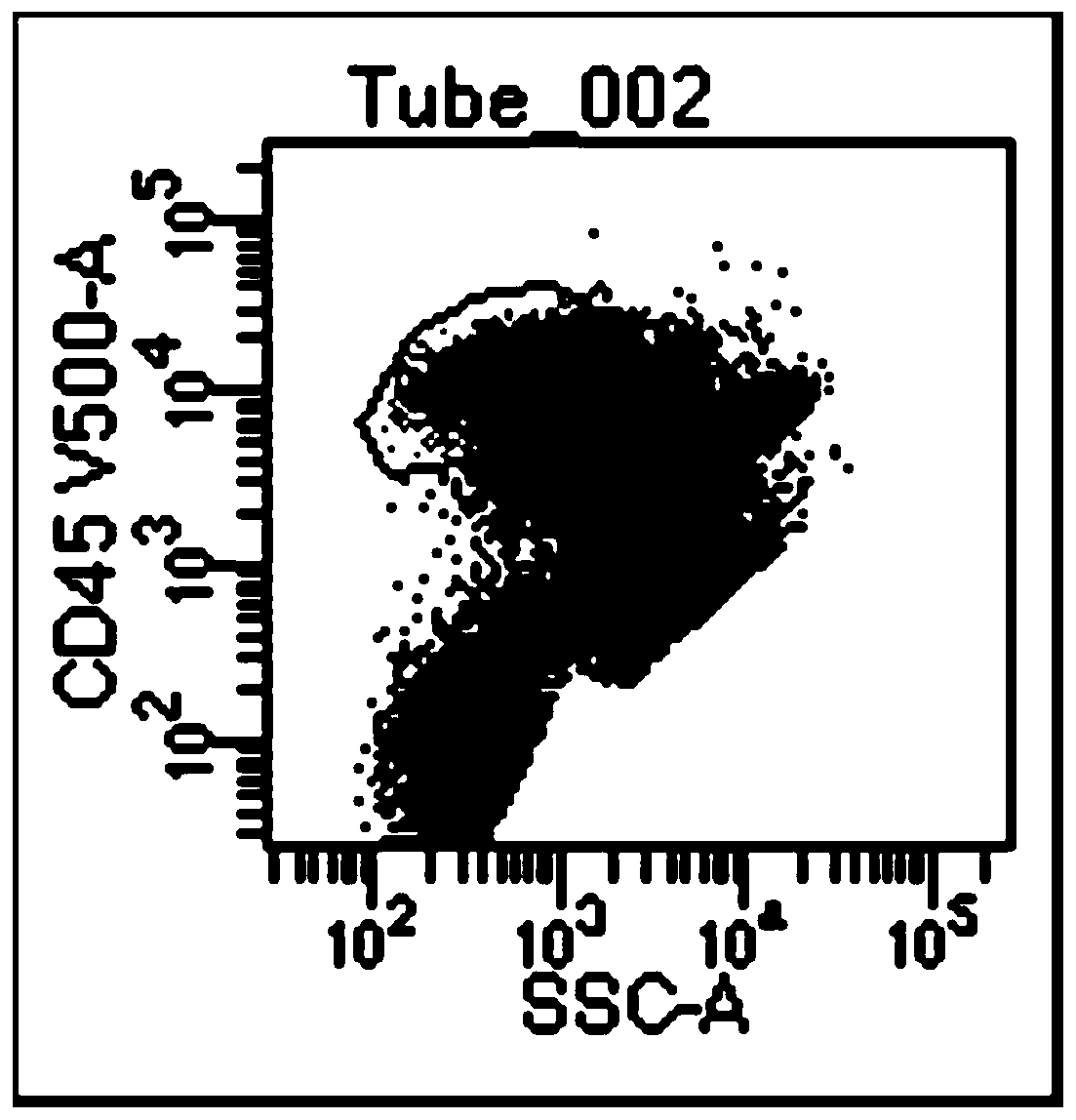
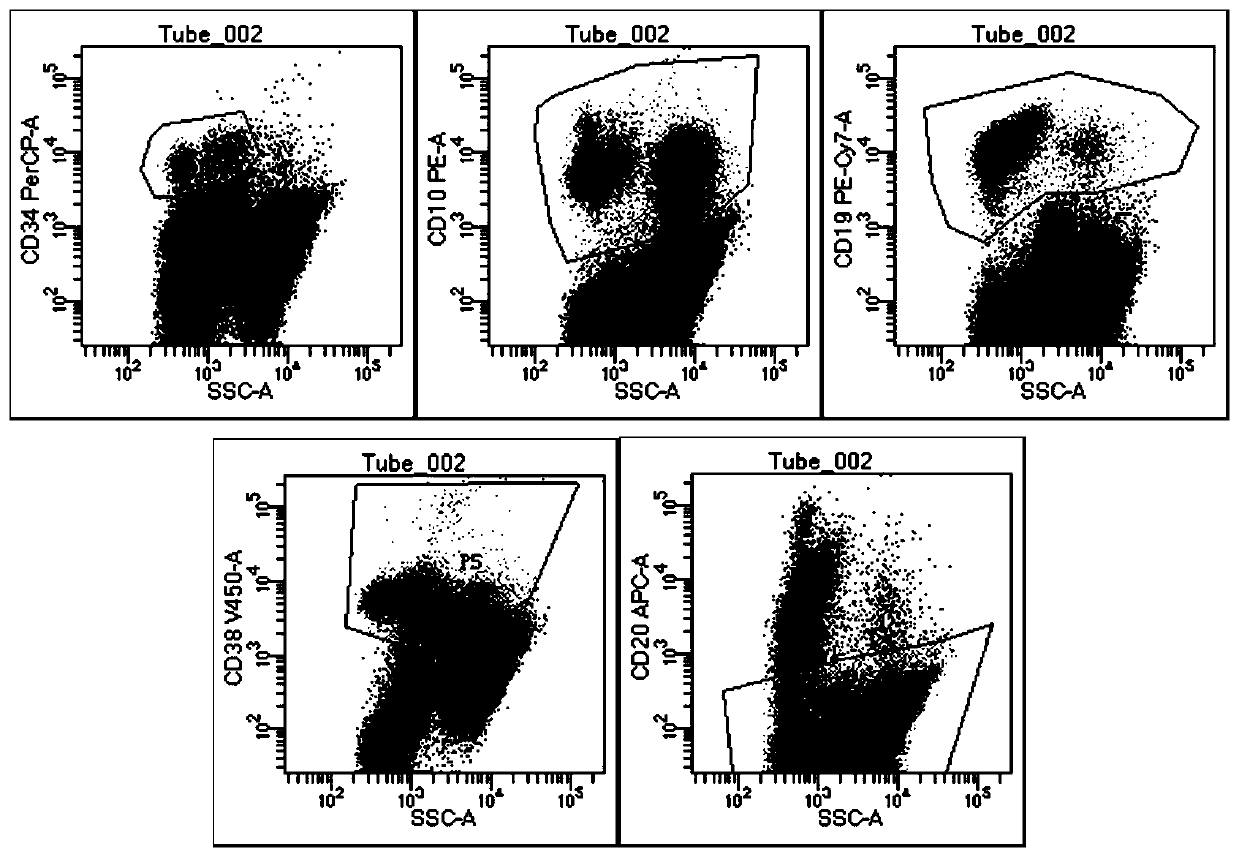
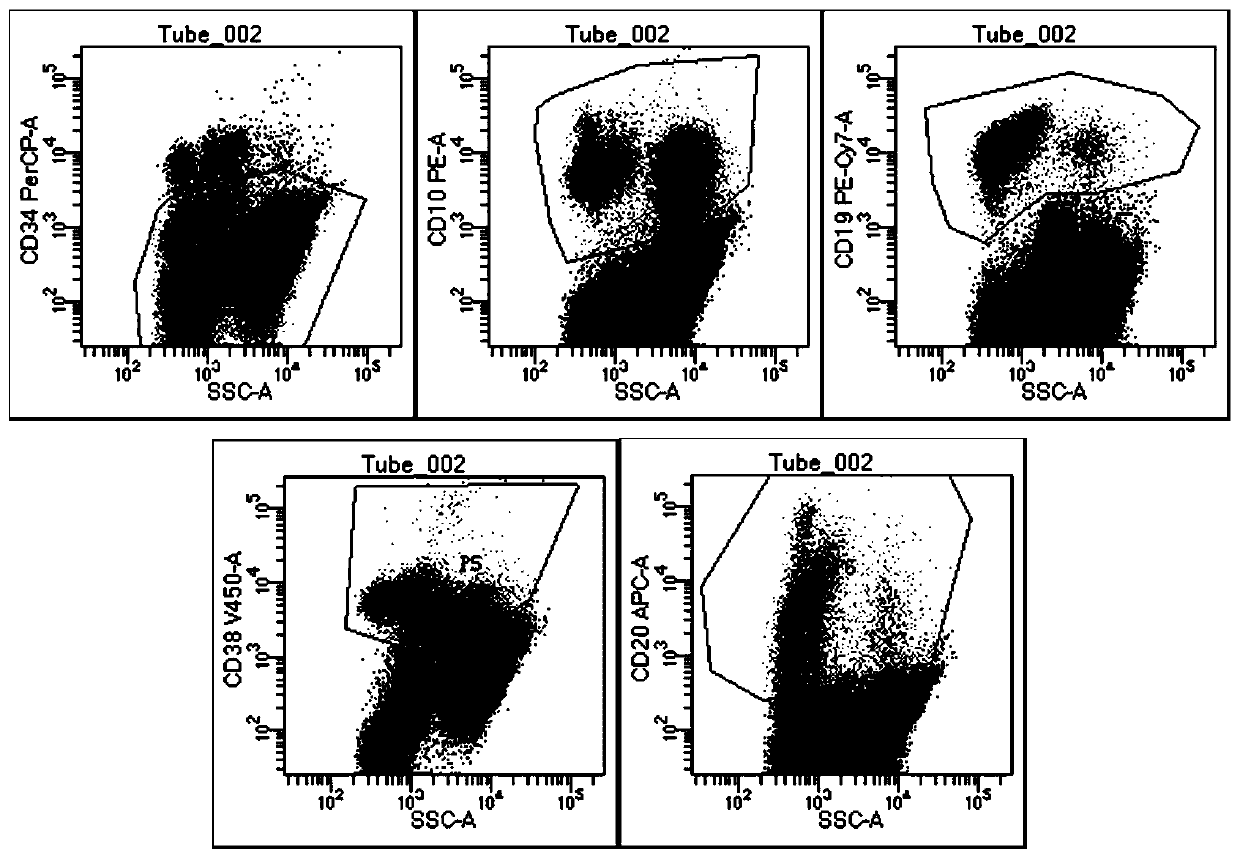
[0068] (1) Take the numbers of two flow tubes, one is the control tube and the other is the detection tube, and the antibodies CD10-PE, CD19-APC, CD20-APC-cy7, CD34-PerCP, CD38-V450, CD45-V500 Add to the detection tube to ensure that the mass ratio of CD10-PE, CD19-APC, CD20-APC-cy7, CD34-PerCP, CD38-V450, and CD45-V500 is 2:5:5:13:20:40. The total amount of antibodies is 16 μl, including 4 μl of CD10-PE, 2 μl of CD19-APC, 2 μl of CD20-APC-cy7, 4 μl of CD34-PerCP, 2 μl of CD38-V450, and 2 μl of CD45-V500. The antibody can be pre-diluted with antibody dilution reagent; no antibody is added to the control tube.
[0069] (2) Dilute the sample with PBS so that the cell concentration of the sample is 2×10 6 / mL, respectively add 500 μl of diluted sample i...
Embodiment 3
[0077] Example 3 Acute B-lymphocytic Leukemia Minimal Residue Detection Kit and Application of Its Detection Method in Clinical Sample Detection
[0078] The detection kit of Example 1 and the detection method of Example 2 were used to detect 127 clinical samples from different patients with acute B lymphocytic leukemia, such as bone marrow and blood, and each patient's clinical sample detection was provided with a test group and Control group, test group adopt the test kit of embodiment 1 and the detection method of embodiment 2, control group adopt contrast kit and contrast detection method to detect.
[0079] The only difference between the control kit and the kit of Example 1 is that the antibody composition is different. The components of the antibody composition of the control kit are as follows: CD10-PE, CD19-APC, CD34-PerCP, CD38-V450, CD45-V500; The difference between the detection method and the detection method in Example 2 is that the antibody composition added in ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com