Polyamine micromolecular developer, production method and application thereof
A technology of small molecule compound and PET imaging agent, which is applied in the field of preparing PET imaging agent and cell apoptosis-coupled polyvalent polyamine small molecule compound, can solve the problem of poor early imaging effect and difficult to detect apoptotic cells , large molecular weight, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0122] 【Example 1】 18 F-FEN-DPAZn2 and 18 Synthesis of F-FB-DPAZn2 precursor
[0123] 1. Synthesis of dimethyl 15-hydroxyisophthalate (1)
[0124] 5-Hydroxyisophthalic acid (10 g, 54.9 mmol) was dissolved in 100 mL of methanol, 5 mL of concentrated sulfuric acid was added, and heated under reflux for 12 hours. The system was cooled, and a white solid was precipitated, filtered, the filter cake was washed with methanol, and dried to obtain 10.93 g of a white solid, with a yield of 94%. Melting point: 102-104°C.
[0125] 1.23, Synthesis of 5-dimethylolphenol (2)
[0126] Dimethyl 5-hydroxyisophthalate (1) (5 g, 23.8 mmol) was added to 100 mL of dry tetrahydrofuran, and lithium aluminum hydride (2.7 g, 71.4 mmol) was added in batches, and heated to reflux for 3 hours. The system was cooled to room temperature, and distilled water was added dropwise until no bubbles were generated. Add dilute hydrochloric acid to adjust the pH to 3, extract with ethyl acetate (50 mL×3), comb...
Embodiment 2
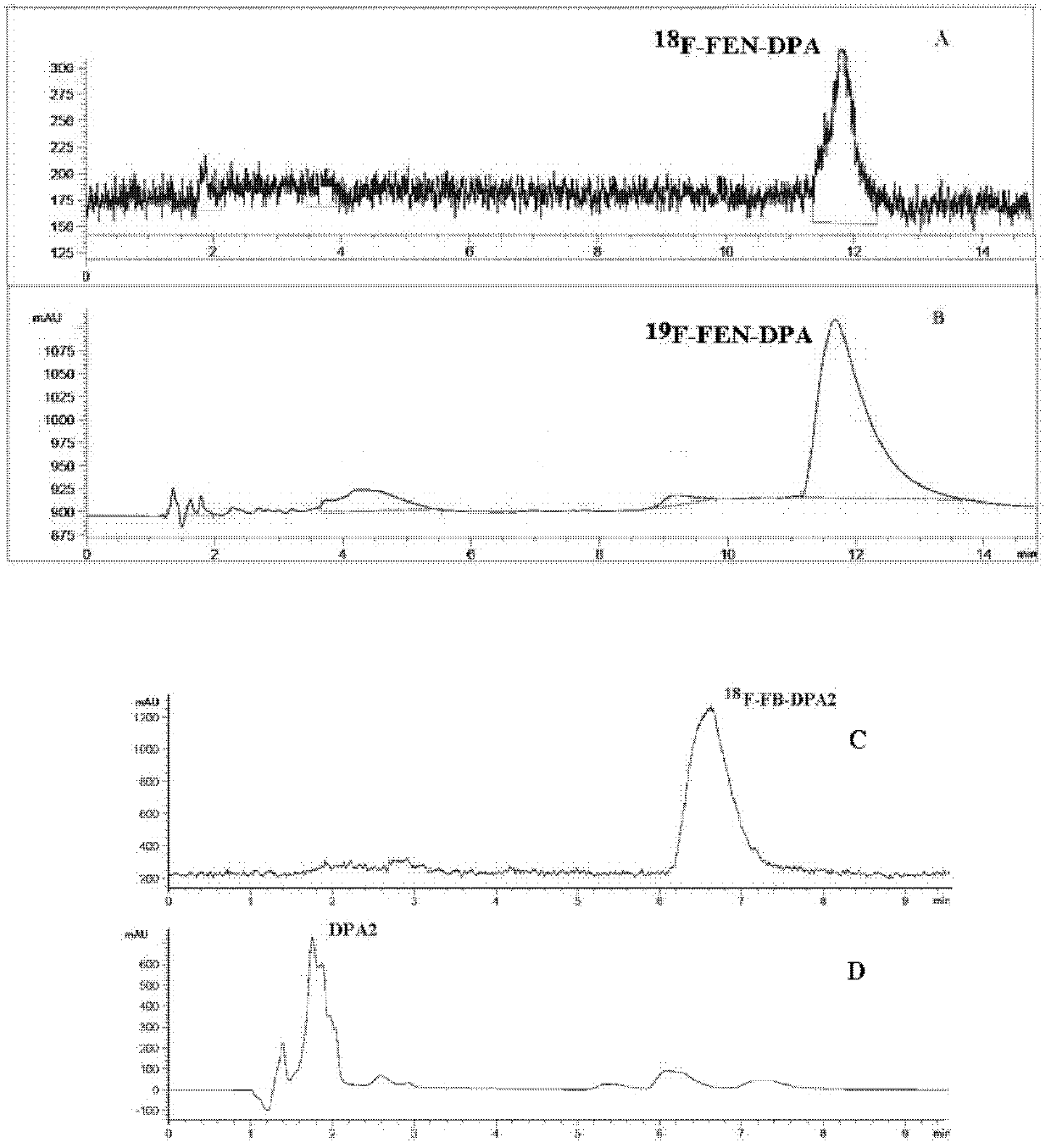
[0137] [Example 2] 18 F-FEN-DPAZn2 and 18 Radiosynthesis of F-FB-DPAZn2
[0138] 2.1 18 Radiochemical synthesis of F-FEN-DPA2 [see the reaction formula of compound (7) into compound (8)]
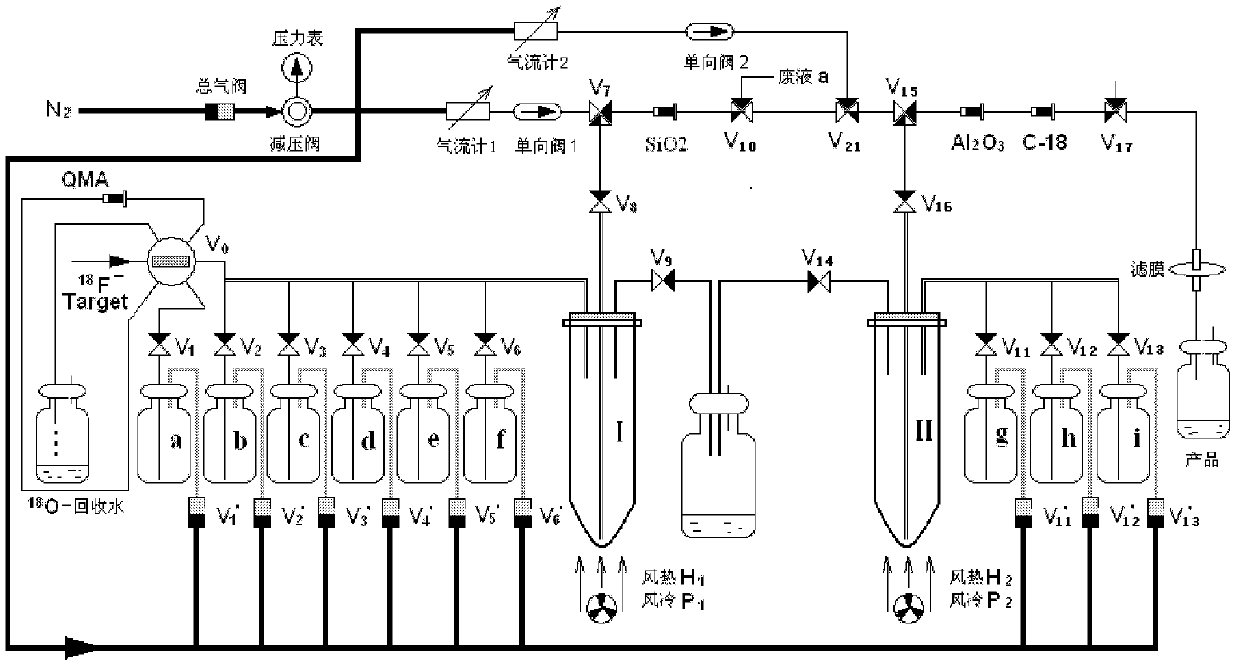
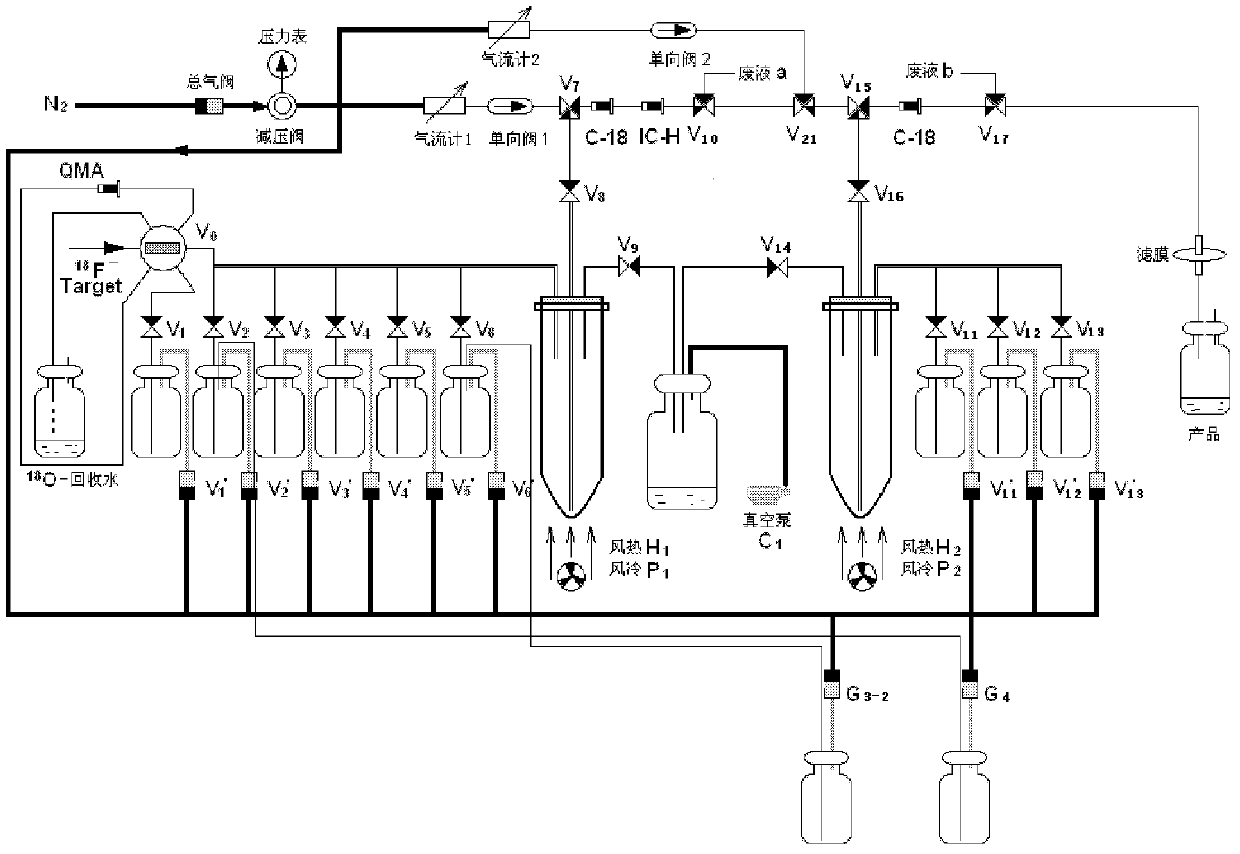
[0139] Such as figure 1 As shown, with PET-MF-2V-IT-I type fluorine-18 multifunctional synthesis module (produced by Paite (Beijing) Technology Co., Ltd., Tang G*, Tang X, Wen F, Wang M, Li B.A facile and rapid automated synthesis of 3'-deoxy-3'-[ 18 F]fluorothymidine. Appl Radiat Isot, 2010, 68: 1734-1739.) carried out 18 F mark, the specific mark operation is as follows:
[0140] 2.1.1 18 f - Accelerator produced by Cyclone 10 / 5 type IBA, through 18 O(p,n) 18 F reaction production. Apply 2.0mL H 2 18 O target, produced by continuously bombarding the target with 10.5MeV, 25μA proton beam for 10-60min 18 f - , take a certain activity 18 f - The target water passes through the QMA column;
[0141] 2.1.2 18 F - After being captured by QMA, use 1.5 mL of K in bottle a 2 CO ...
Embodiment 3
[0172] [Example 3] organic synthesis of fluorescent imaging agent Dansy1-DPA2 and Dansy1-DPAZn2
[0173] Dansylation-N-(2-{2-[2-(3,5-di-N,N-bis(2-methylpyridine)aminomethyl-phenoxy)ethoxy]-ethoxy Base} ethyl) amino) (Dansy1-DPA2) [synthetic route see compound (7) into (10) part].
[0174] (2-{2-[2-(3,5-di-N,N-bis(2-picoline)aminomethyl-phenoxy)ethoxy]-ethoxy}ethyl)- Amino (7) (300mg, 0.46mmol) was dissolved in 15mL of acetonitrile, added triethylamine (56.7mg, 0.56mmol, 1.2eq.), added 20ml of dry dichloromethane, cooled to 0°C in an ice bath, dansyl chloride (149mg, 0.56mmol, 1.2eq.), under argon protection, stirred at room temperature for 3h. TLC monitoring, the reaction has been completed. First, the solvent was evaporated, 20ml of distilled water was added, extracted with ethyl acetate (20ml×4), the organic phases were combined, dried over anhydrous sodium sulfate, concentrated, and then column chromatographed (ethyl acetate:methanol ammonia=15:1) to obtain Green oily f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com