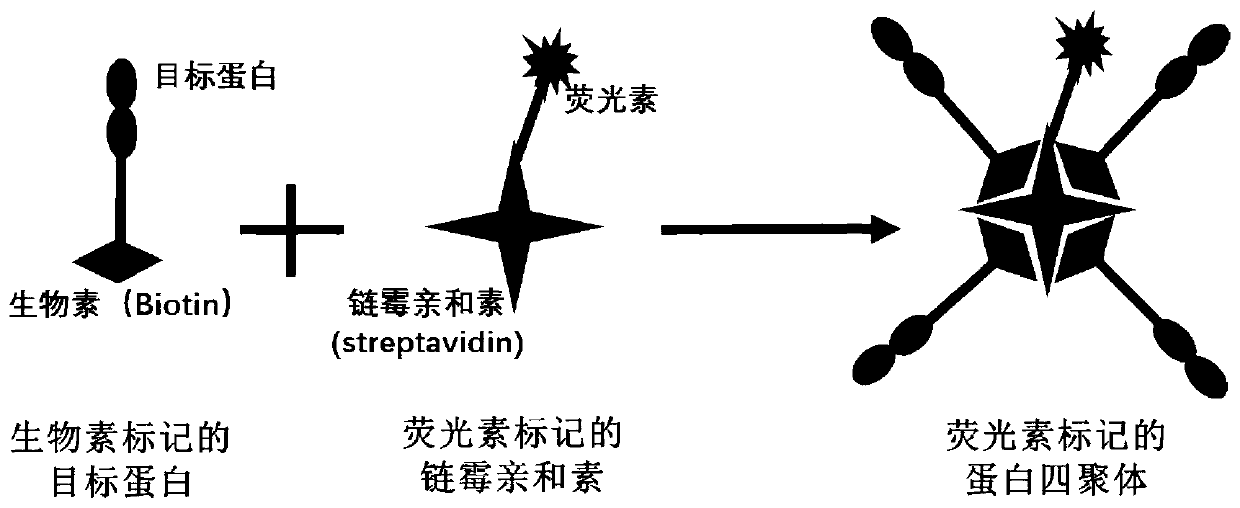
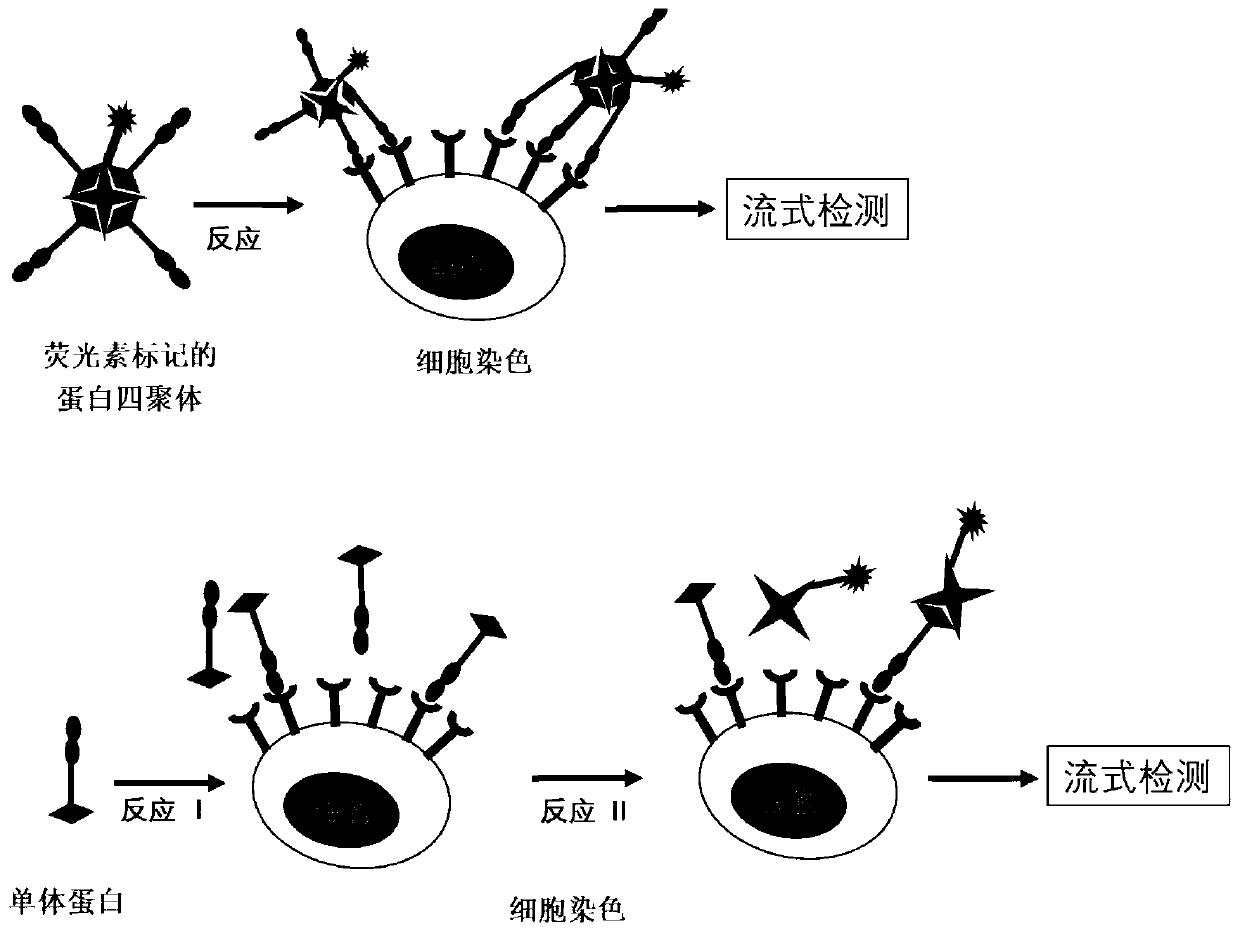
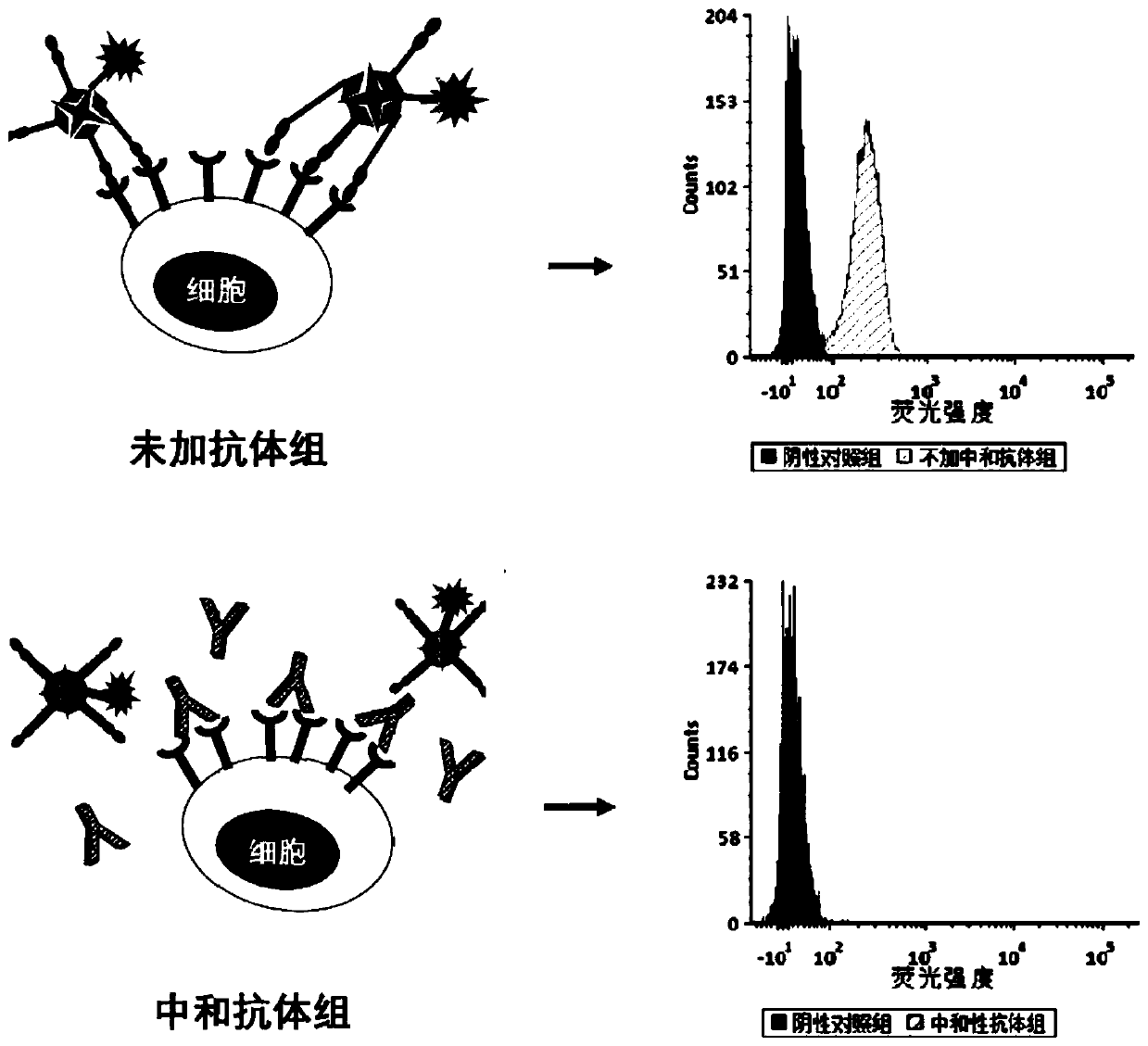
Fluorescein marked protein tetramer as well as preparation method and application thereof
A fluorescein-labeled, tetramer technology, applied in the field of fluorescein-labeled protein tetramer and its preparation, can solve the problems of poor detection sensitivity of CAR-T target protein, inability to detect whether CAR can be recognized, etc. The effect of improved detection sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1. Preparation and Application of Immune Checkpoint Protein Tetramer (PDCD1 / PD1 C-Fc-avi-PE Tetramer)
[0039] 1. Preparation of Biotinylated Human PD-1 / PDCD1Avi Tag Protein
[0040] 1.1. Reagents and instruments
[0041] TransT1 competent cells (full gold, CD501-01); TransT1 competent cells (ThermoFisher, C505003); tryptone (Sigma, T7293-1KG), yeast extract (Sigma, Y1625-1KG); agar (Sigma, A7002 -1KG); Max plasma Kit (promega, A2492); Bacterial shaker (Uno, HN211B); Bacterial incubator (Tianjin Tester, DH3600B) Nanodrop 2000; Expi293F cells (Invitrogen, Cat#R790-07); cells Expression medium, prepared by the laboratory; Polyethyleneimine, PEI (Polysciences, Cat#23966). Cell shaker incubator (Uno, HNY-2102)
[0042] 1.2. Solution preparation
[0043] 1.2.1.LB medium: tryptone 10g, yeast extract 5g, sodium chloride 10g, add water to 1000ml, autoclave, cool to room temperature, add Amp, the final concentration is 100ug / ml, set aside.
[0044] 1.2.2.LB solid me...
Embodiment 2
[0099] Example 2. Preparation and application of CAR-T target protein tetramer (CD19 Fc-Avi-PE tetramer)
[0100] 1. Preparation of Biotinylated Human CD19 FcAvi Tag Protein
[0101] 1.1. Reagents and instruments
[0102] TransT1 competent cells (full gold, CD501-01); TransT1 competent cells (ThermoFisher, C505003); tryptone (Sigma, T7293-1KG), yeast extract (Sigma, Y1625-1KG); agar (Sigma, A7002 -1KG); Max plasma Kit (promega, A2492); Bacterial shaker (Uno, HN211B); Bacterial incubator (Tianjin Tester, DH3600B) Nanodrop 2000; Expi293F cells (Invitrogen, Cat#R790-07); cells Expression medium, prepared by the laboratory; Polyethyleneimine, PEI (Polysciences, Cat#23966). Cell shaker incubator (Uno, HNY-2102).
[0103] 1.2. Solution preparation
[0104] 1.2.1.LB medium: tryptone 10g, yeast extract 5g, sodium chloride 10g, add water to 1000ml, autoclave, cool to room temperature, add Amp, the final concentration is 100ug / ml, set aside.
[0105]1.2.2.LB solid medium: tryptone ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com