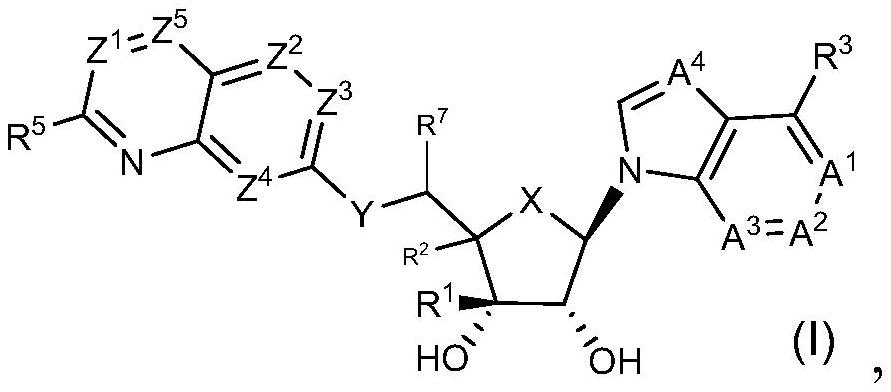
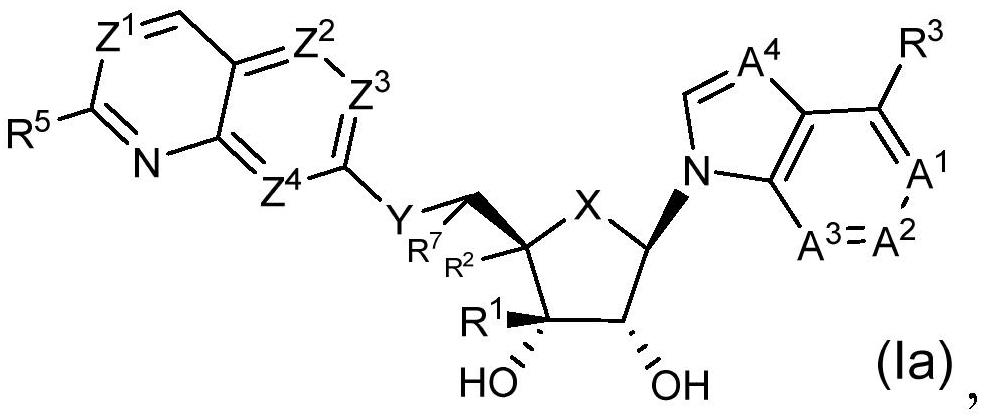
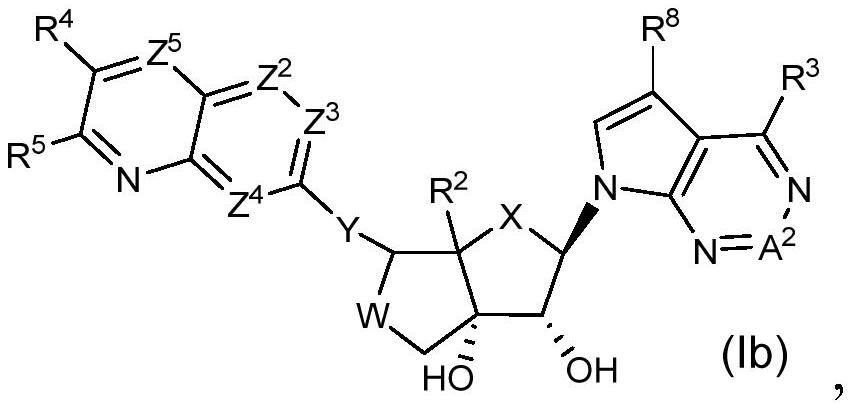
Prmt5 inhibitors
A C2H5, halogen technology for the genetic persistence of sickle cell and fetal hemoglobin mutations, PRMT5 inhibitors, the treatment of cancer, the preparation of compounds of formula I, which can solve elusive problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific example
[0350] Some specific examples of tyrosine kinase inhibitors: include N-(trifluoromethylphenyl)-5-methylisoxazole-4-carboxamide, 3-[(2,4-dimethylpyrrole-5 -yl)methylene)indol-2-one, 17-(allylamino)-17-desmethoxygeldanamycin, 4-(3-chloro-4-fluorophenylamino)- 7-Methoxy-6-[3-(4-morpholinyl)propoxy]quinazoline, N-(3-ethynylphenyl)-6,7-bis(2-methoxyethoxy base)-4-quinazolinamine, BIBX1382, 2,3,9,10,11,12-hexahydro-10-(hydroxymethyl)-10-hydroxy-9-methyl-9,12-epoxy -1H-Diindolo[1,2,3-fg:3',2',1'-kl]pyrrolo[3,4-i][1,6]benzodiazon-1-one, SH268 , genistein, STI571, CEP2563, 4-(3-chlorophenylamino)-5,6-dimethyl-7H-pyrrolo[2,3-d]pyrimidine methanesulfonate, 4-(3- Bromo-4-hydroxyphenyl)amino-6,7-dimethoxyquinazoline, 4-(4'-hydroxyphenyl)amino-6,7-dimethoxyquinazoline, SU6668, STI571A, N-4-Chlorophenyl-4-(4-pyridylmethyl)-1-phthalazinamine and EMD121974.
[0351] Combinations with compounds other than anticancer compounds are also included in this method. For example, combinations of c...
Embodiment 1
[0816] (1R, 2S, 3R, 5R)-5-(((2-amino-3-bromoquinolin-7-yl)oxy)methyl)-3-(4-chloro-7H-pyrrolo [2,3-d]pyrimidin-7-yl)-1-methylcyclopentane-1,2-diol
[0817]
[0818] step 1: ((3aR, 4R, 6R, 6aS)-6-(4-chloro-7H-pyrrolo[2,3-d]pyrimidin-7-yl)-2,2,3a-trimethyltetrahydro-3aH -Cyclopenta[d][1,3]dioxol-4-yl)methanol (1.2g, 3.6mmol), 2-amino-3-bromoquinolin-7-ol (0.934 g, 3.91 mmol) and triphenylphosphine (1.86 g, 7.10 mmol) were coevaporated with dry toluene (three times, 10 mL each) and then redissolved in anhydrous THF (20 mL). The reaction mixture was cooled to 0°C, and (E)-diisopropyldiazene-1,2-dicarboxylate (1.44 g, 7.10 mmol) was added dropwise at 0°C. The mixture was naturally warmed to room temperature, and the mixture was stirred at room temperature for 2 hours. The reaction mixture was concentrated under reduced pressure. The residue was purified by column chromatography on silica gel (0-10% MeOH in DCM). Fractions containing the desired product were combined and...
Embodiment 2
[0821] (1R,2S,3R,5R)-5-(((2-amino-3-bromoquinolin-7-yl)oxy)methyl)-3-(4-amino-7H-pyrrole And[2,3-d]pyrimidin-7-yl)-1-methylcyclopentane-1,2-diol
[0822]
[0823] To a sealed tube (10 mL) was added (1R,2S,3R,5R)-5-(((2-amino-3-bromoquinolin-7-yl)oxy)methyl)-3-( 4-Chloro-7H-pyrrolo[2,3-d]pyrimidin-7-yl)-1-methylcyclopentane-1,2-diol (350mg, 0.675mmol), 1,4-dioxane (3mL) and NH 3 ·H 2 O (5 mL; 25%-28% w / w). The reaction mixture was sealed tightly and then stirred at 90°C for 16 hours. The reaction mixture was concentrated under reduced pressure. The residue was purified by reverse phase HPLC (0-45% acetonitrile / water) to give (1R,2S,3R,5R)-5-(((2-amino-3-bromoquinolin-7-yl)oxy) Methyl)-3-(4-amino-7H-pyrrolo[2,3-d]pyrimidin-7-yl)-1-methylcyclopentane-1,2-diol. MS 499,501 (M+1,M+3). 1 H NMR (400MHz, DMSO-d 6 )δ8.28(s,1H),8.02(s,1H),7.58(d,J=8.7Hz,1H),7.30(d,J=3.6Hz,1H),6.96–6.89(m,4H), 6.57–6.53(m,3H),4.97–4.91(m,2H),4.41(s,1H),4.19–4.10(m,3H),2.45–2.37(m,2H),1.72–...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com