Stable tulobuterol percutaneous absorption preparation
A technology of tulobuterol and preparations, which can be applied to medical preparations with non-active ingredients, medical preparations containing active ingredients, respiratory diseases, etc. Low, unable to maintain good efficacy and other problems, to achieve the effect of reducing discomfort, superior adhesion, and simple structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1-5
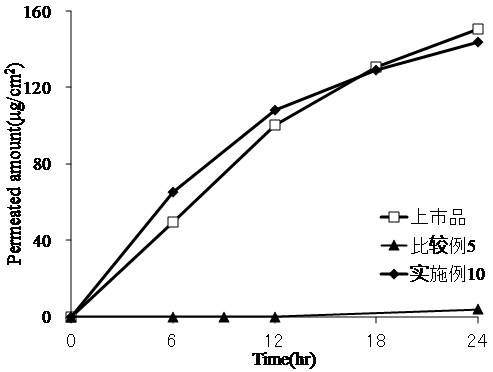
[0029] Embodiment 1-5, comparative example 1-4
[0030] Uniformly mix 39 parts by weight of 2-ethylhexyl methacrylate, 6.5 parts by weight of dodecyl methacrylate, 4.5 parts by weight of 2-ethylhexyl acrylate and 30 parts by weight of ethyl acetate, add azobisiso After 0.6 parts by weight of butyronitrile, a polymerization reaction was carried out under a nitrogen flow environment at 65° C. to prepare an alkyl (meth)acrylate polymer (referred to as binder A). A drug-containing coating solution is prepared by adding a quantitative amount of tulobuterol and an amine compound (ammonia water, diethanolamine, ethylenediamine, ammonium acetate, ammonium chloride) into a quantitative polymer ethyl acetate solution. Coat the coating solution on the release film, and dry it at 70°C for 8 minutes to make the thickness of the adhesive layer reach about 46 μm. A polyethylene terephthalate film is compounded on the surface of the adhesive layer to obtain the percutaneous absorption prepar...
Embodiment 6-10
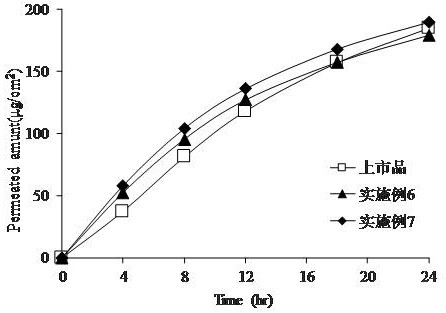
[0041] Examples 6-10, Comparative Example 5 and Reference Example (marketed product)
[0042] Add 4.0 to 4.8 parts by weight of tulobuterol, 0.05 parts by weight of ammonia water, 1.0 parts by weight of Span 80 and 3.0 parts by weight of DL-α-tocopherol in the quantitative binder A ethyl acetate solution, Mix to obtain the drug-containing coating solution. Apply the coating solution on the surface of the release film, and dry it at 70°C for 8 minutes to make the thickness of the adhesive layer reach about 46 μm. A polyethylene terephthalate film is compounded on the surface of the adhesive layer, namely the percutaneous absorption preparation of dertulobuterol. As a comparative example, a tulobuterol percutaneous absorption preparation was prepared using a carboxyl group-containing acrylic binder. Tables 4 and 5 are the formulations (by weight) of Examples 6-10 and Comparative Example 5.
[0043] 【Table 4】
[0044]
[0045] 【table 5】
[0046]
[0047] A transdermal ...
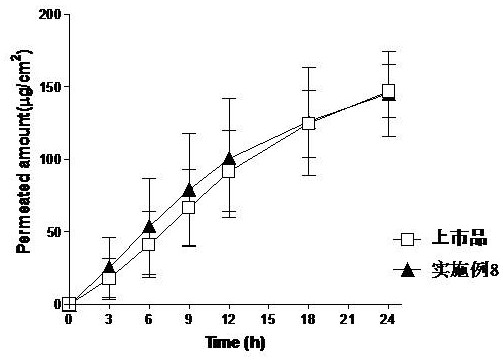
Embodiment 8
[0061] Embodiment 8: There is no residual glue phenomenon in the sticking part, there is no peeling off around the patch, and the peeling strength is weak;
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com