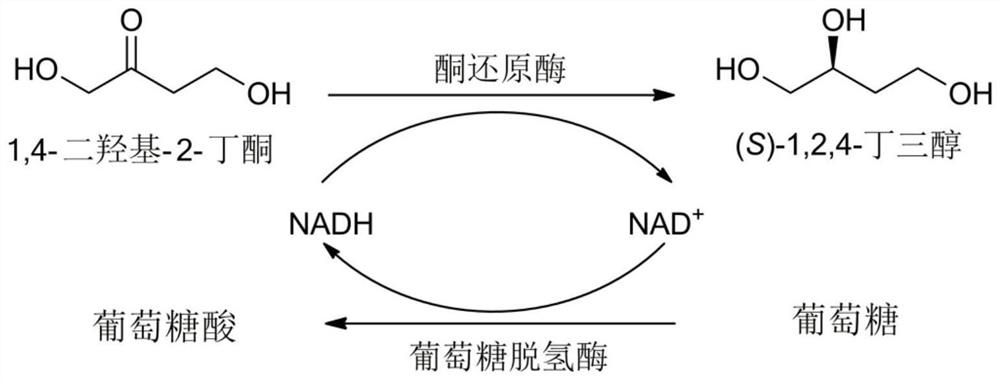
A method for enzymatically synthesizing (s)-1,2,4-butanetriol
An enzymatic synthesis, butanetriol technology, applied in the directions of oxidoreductase, fermentation, bulk chemical production, etc., can solve the problems of unfavorable industrialization, high cost, large pollution, etc., and achieves small amount of organic solvent, mild reaction conditions, The effect of green and environmental protection of the reaction system
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Preparation of recombinant ketoreductase:
[0038] The gene sequence of the whole gene synthesis is shown as SEQ ID No.1.
[0039] The resulting gene fragment was double-digested with restriction enzymes NdeI and HindIII for 12h; the pET30a(+) vector was double-digested with restriction enzymes NdeI and HindIII for 12h to obtain a linear pET30a(+) vector;
[0040] The double digested gene fragment was ligated with the linear pET30a(+) vector with DNA ligase overnight at 16°C, and the ligated product was transformed into Escherichia coli JM109 competent cells;
[0041] The positive recombinants were screened on the resistance plate containing kanamycin, and the recombinant expression vector was obtained after the positive transformants were picked and sequenced and identified;
[0042] After the recombinant expression vector containing the target gene is transferred into Escherichia coli BL21 (DE3) competent cells, the genetically engineered bacteria that can induce the...
Embodiment 2
[0045] Preparation of (S)-1,2,4-butanetriol:
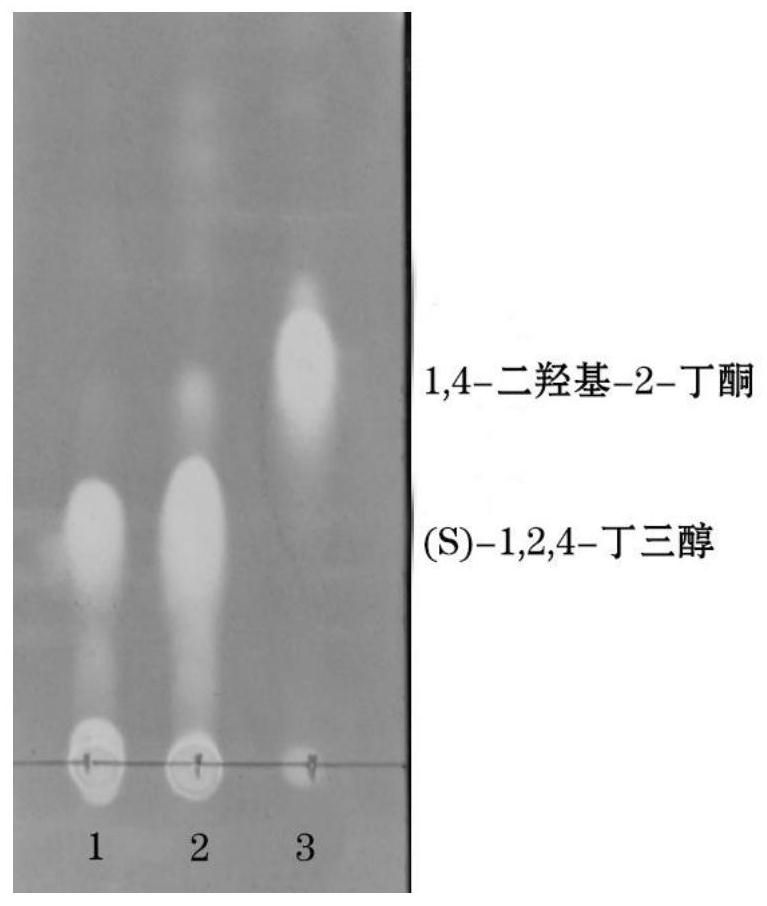
[0046] Add 100g 1,4-dihydroxy-2-butanone, 40g wet bacteria containing recombinant ketoreductase, 0.2g coenzyme NAD+, 4g glucose dehydrogenase enzyme powder, 120g Anhydrous glucose, dilute to 1L. The reduction reaction was carried out in a water bath at 30°C. During the reaction, 3 mol / L sodium hydroxide solution was added to maintain the pH of the reaction solution at 6.5. TLC detected that the raw material point disappeared, that is, the reaction was complete.
[0047] After the reaction solution was kept at 80°C for 1 hour, it was left to cool in ice water and a large amount of solid precipitated out, which was filtered under reduced pressure.
[0048] The solid was washed twice with 500ml of absolute ethanol, the filtrate and the washings were combined, and the solvent was distilled off under reduced pressure to obtain 118g of a yellow-brown oily product, which was the crude product of (S)-1,2,4-butanetriol.
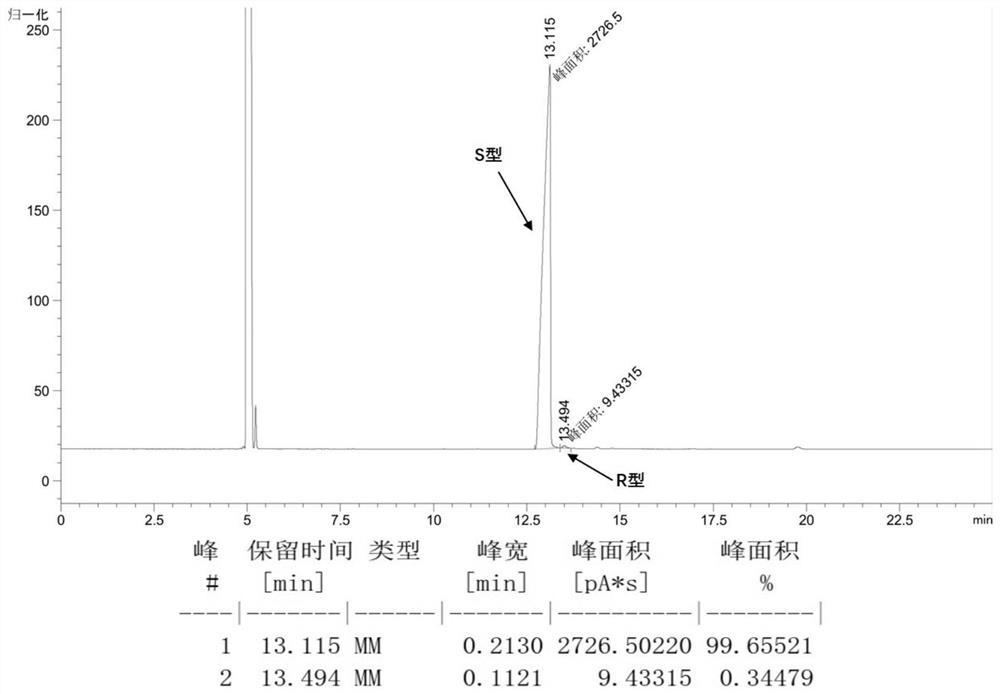
[0049] The crud...
Embodiment 3
[0051] Preparation of (S)-1,2,4-butanetriol:
[0052] Add 150g 1,4-dihydroxy-2-butanone, 60g wet bacteria containing recombinant ketoreductase, 0.3g coenzyme NAD+, 6g glucose dehydrogenase enzyme powder, 180g anhydrous glucose, dilute to 1L. Carry out the reduction reaction in a water bath at 30°C;
[0053] During the reaction process, 3 mol / L sodium hydroxide solution was added to maintain the pH of the reaction solution at 6.5, and TLC detected that the raw material point disappeared, that is, the reaction was completed.
[0054] After the reaction solution was kept at 80°C for 1 hour, it was left to cool in ice water and a large amount of solid precipitated out, which was filtered under reduced pressure.
[0055] The solid was washed twice with 500ml of absolute ethanol, the filtrate and washings were combined, and the solvent was distilled off under reduced pressure to obtain 166g of a yellow-brown oily product, which was the crude product of (S)-1,2,4-butanetriol.
[0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com