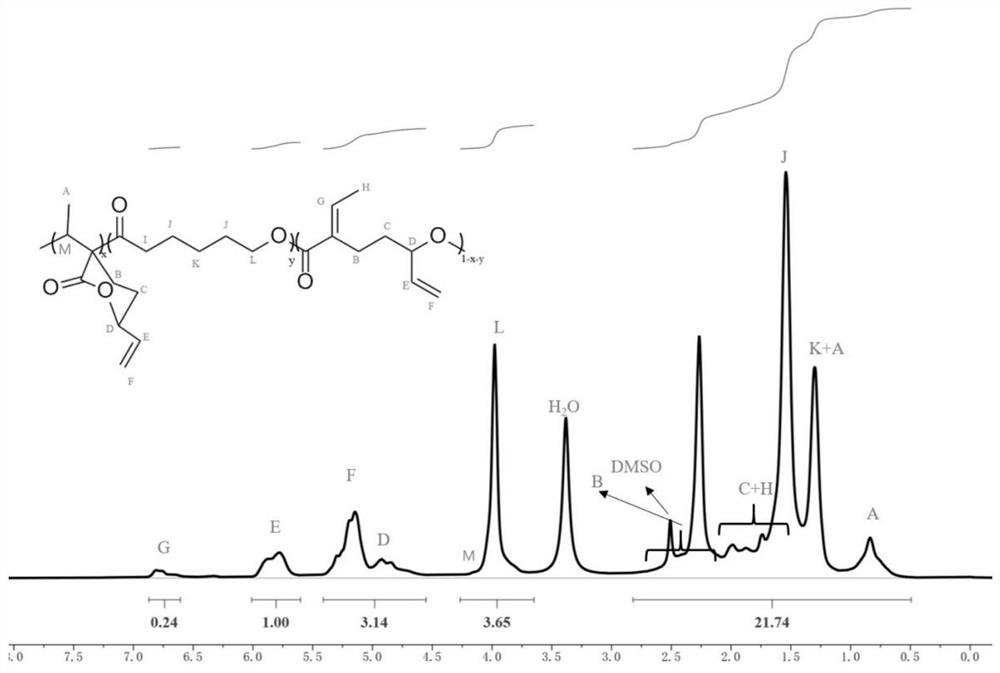
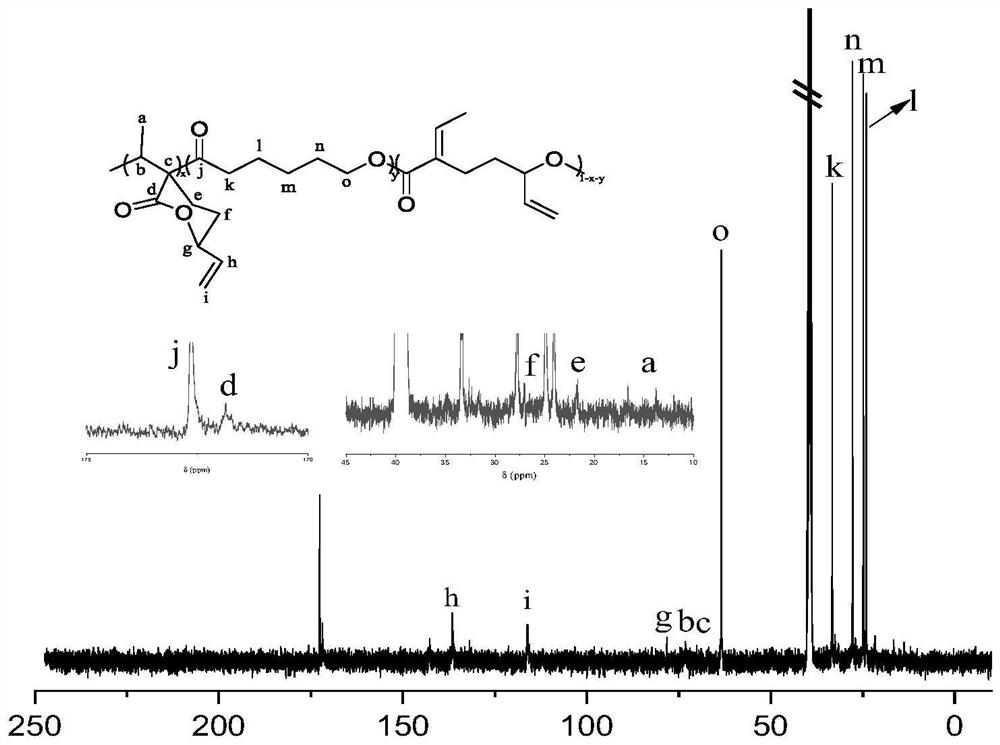
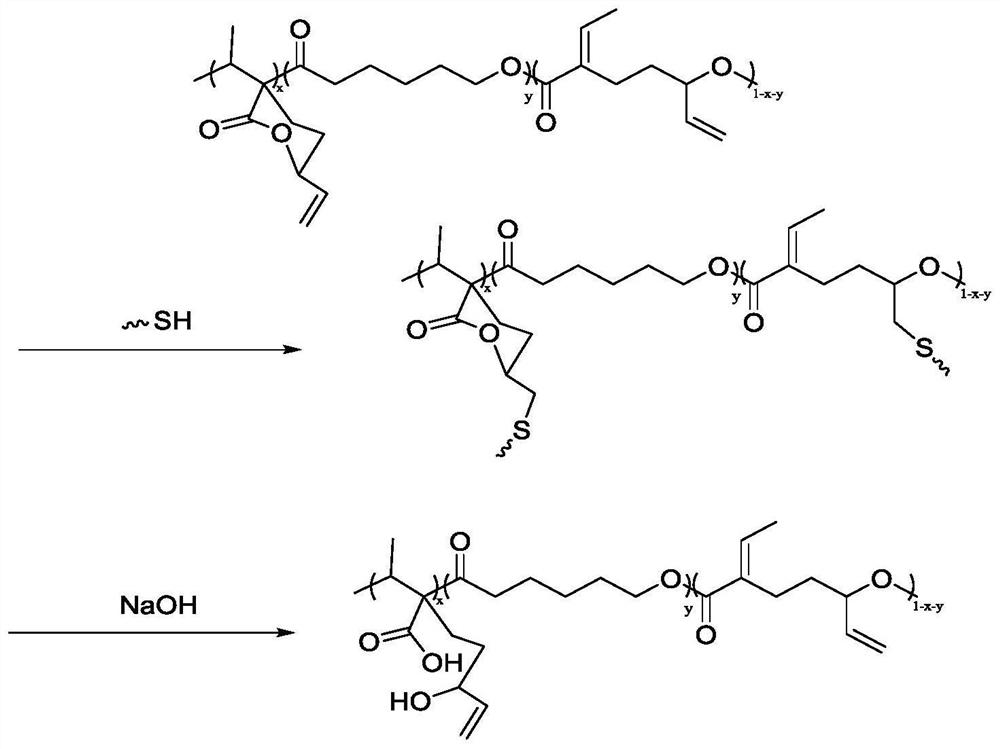
Preparation of functionalized polyesters and polycarbonates by copolymerization of polysubstituted six-membered ring lactones and methods for their post-modification
A polycarbonate and copolymerization technology, which is used in the preparation of functionalized polyesters and polycarbonates and their post-modification through the copolymerization of multi-substituted six-membered ring lactones, and can solve the modification of polyester or polycarbonate. Limited, low content of functional groups, complex process and other problems, to achieve the effect of abundant sites, changing physical and chemical properties, and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] The polymerization reaction adopts Schlenk technique. The glass instruments used were pumped and baked in an argon atmosphere for 3 times to achieve the purpose of removing water and oxygen. Draw 0.2mL tetrahydrofuran solution of sodium 2,6-di-tert-butyl-4-methylphenolate into the polymerization bottle after pumping and roasting, remove the THF solvent under reduced pressure, fill with argon, weigh the bottle, and obtain the polymerization The quality of sodium 2,6-di-tert-butyl-4-methylphenate (catalyst) in the bottle is 0.053 g. Add 0.323g EVL, 0.276gε-CL and 2.2mL of anhydrous toluene to another polymerization bottle that has been pumped and baked, stir and mix evenly, then add the uniformly mixed monomer solution into the polymerization bottle containing the catalyst, set React in an oil bath at 25°C for 5 hours. The resulting polymer was first diluted with THF and then precipitated once with ether. The precipitate was dissolved in THF and precipitated in n-hexan...
Embodiment 2
[0062] Other polymerization conditions were the same as in Example 1, and the polymerization reaction time was extended to 12 hours to obtain a crosslinked network of copolyester. The cross-linked network swells in THF solution and increases in volume. It shows that the cross-linked network of the polymer can be obtained by the in-situ one-step method of the copolymer.
Embodiment 3
[0064] Other polymerization conditions are the same as in Example 1, except that δ-VL is used as the polyester monomer. The yield of the obtained copolymer was 66%, the number average molecular weight of the copolymer measured by GPC was 12.1 kDa, and the molecular weight distribution was 2.99. It shows that different lactone monomers can also obtain polyesters with modifiable sites.
PUM
| Property | Measurement | Unit |
|---|---|---|
| number average molecular weight | aaaaa | aaaaa |
| number average molecular weight | aaaaa | aaaaa |
| number average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com