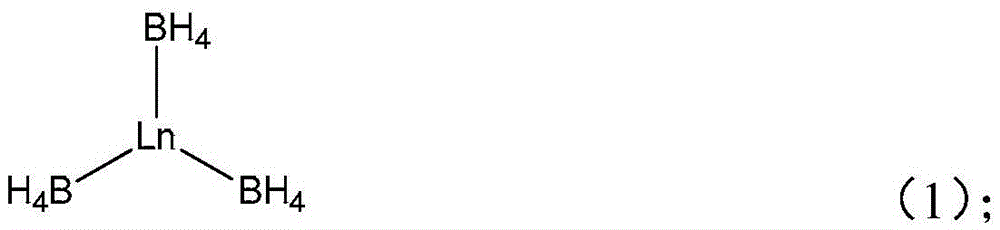Synthetic method of rare earth catalyst catalyzed clustering peptide and synthesized clustering peptide
A rare earth catalyst and a synthesis method technology are applied in the field of synthetic cluster peptides and the synthesis of cluster peptides to achieve the effects of stable properties, simple and easy synthesis process, and large-scale industrial production.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
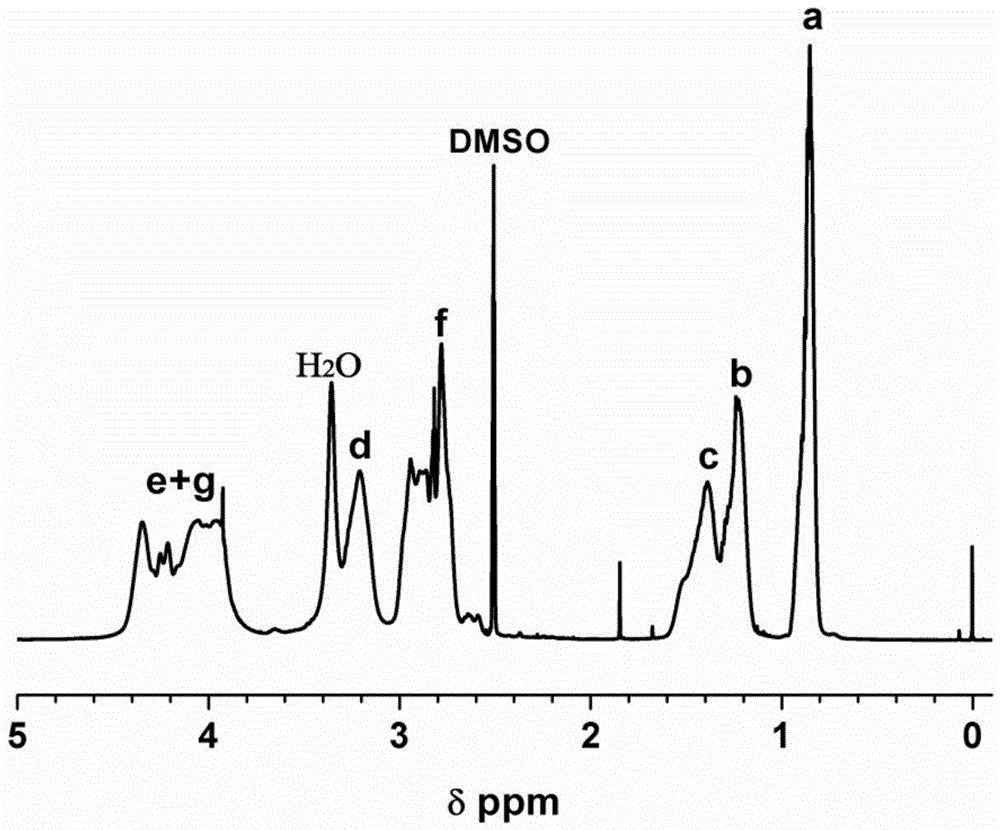
[0036] Add 0.429g (3.73mmol) of N Methyl-NCA (that is, R in formula (4) is methyl), dissolved with 7.5mL N,N-dimethylformamide (DMF), and then added 0.17mLDy (BH 4 ) 3 THF solution (0.022mol / L, 0.00374mmol), N Methyl-NCA and Dy(BH 4 ) 3 The molar ratio is 1000:1, sealed and placed in a 40°C oil bath to react for 4 days. After the reaction, the mixture is poured into ether to precipitate and filtered, and the obtained polymer is vacuum-dried for 4 days to obtain a clustered peptide. The yield 99%. The SEC number-average absolute molecular weight of the obtained polymer was 74.1 kDa, and the molecular weight distribution was 1.42.
Embodiment 2
[0038] Other polymerization conditions are identical with embodiment 1, difference is to use Sc(BH 4 ) 3 Initiate for the initiator N Allyl-NCA (that is, R in the formula (4) is allyl) ring-opening polymerization, the solvent is benzonitrile, N Allyl-NCA and Sc(BH 4 ) 3 The molar ratio was 400:1, and the reaction was carried out in a constant temperature bath at 80° C. for 24 hours, and the yield of the obtained clustered peptide was 92%. The SEC number-average absolute molecular weight of the obtained polymer was 39.0 kDa, and the molecular weight distribution was 1.36.
Embodiment 3
[0040] Other polymerization conditions are identical with embodiment 1, difference is with Nd (BH 4 ) 3 Initiate for the initiator N Oligo(ethyleneglycol)-NCA (that is, R in formula (4) is an oligoethylene glycol group, repeating unit=4), ring-opening polymerization, the solvent is N-methylpyrrolidone (NMP), N Oligo(ethyleneglycol)-NCA and Nd(BH 4 ) 3 The molar ratio was 50:1, and the reaction was carried out in a constant temperature bath at 0°C for 2 days, and the yield of the obtained clustered peptide was 98%. The SEC number-average absolute molecular weight of the obtained polymer was 12.5 kDa, and the molecular weight distribution was 1.19.
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of polymerization | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com