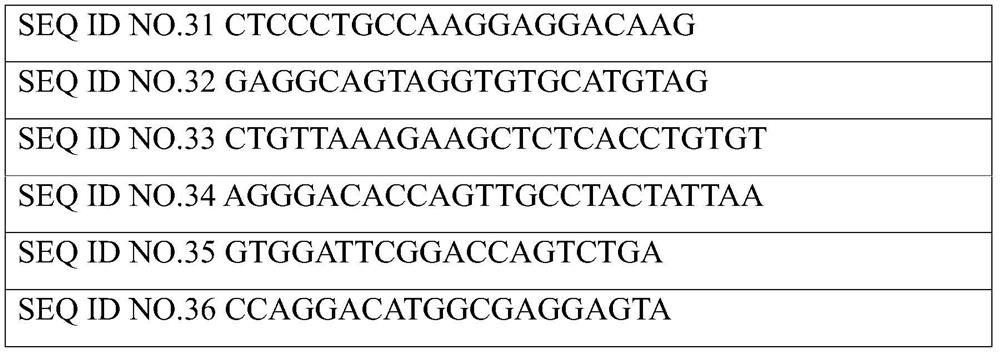Kit for detecting mutation sites of 18 genes related to sensitivity to radiotherapy and chemotherapy of rectal cancer and its application
A mutation site, radiotherapy and chemotherapy technology, applied in the field of biomedical diagnosis, can solve the problems of limited efficiency and difficult markers, and achieve the effect of avoiding complicated detection and saving time and labor costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032]The diagnostic kit for predicting neoadjuvant chemoradiotherapy sensitivity of locally advanced rectal cancer based on gene DNA mutation site of the present invention, said diagnostic kit for predicting neoadjuvant chemoradiotherapy sensitivity of locally advanced rectal cancer based on gene DNA mutation site includes Specific primers for detecting gene mutation sites, the nucleotide sequences of the upstream primers of the specific primers are as shown in SEQ ID NO.1, 3, 5, 7, 9, 11, 13, 15, 17, 19, 21 , 23, 25, 27, 29, 31, 33, 35, the downstream primer nucleotide sequence of the specific primer is as shown in SEQ ID NO.2, 4, 6, 8, 10, 12, 14, 16 , 18, 20, 22, 24, 26, 28, 30, 32, 34, 36.
[0033] 所述的基因DNA突变位点如下:chr6_33052958_C_T、 chr9_125316157_C_T、chr9_123850770_G_A、chr3_122259606_T_C、 chr6_160211646_GTT_、chr1_160920966_C_T、chr8_23294761_T_C、 chr7_122635024_C_T、chr6_56420538_C_T、chr1_232574921_T_C、chr8_21862551_A_G、chr11_66254085_G_T、chr5_55406952_T_C、 chr19_45001346_G...
Embodiment 2
[0048] Collection and preparation of tumor tissue samples
[0049] The inventor collected tumor tissue samples of locally advanced rectal cancer from September 2005 to June 2017, and these groups met the inclusion criteria. The inclusion criteria mainly include four points: 1. Locally advanced rectal patients diagnosed for the first time and who have not received any treatment; 2. Patients receiving standard treatment of neoadjuvant chemoradiotherapy, radical surgery and postoperative adjuvant chemotherapy; Confirmed by examination, TGR grading score, PCR group is TRG 1, that is: pathological complete response (pathological complete response, PCR) after neoadjuvant chemotherapy, and none-PCR group is TRG 2-5, that is: neoadjuvant chemotherapy Group with residual tumor (None-PCR group); 4. Those who are willing to participate in the study and cooperate with follow-up. According to the principle of gender and age matching, the samples of radiochemotherapy sensitive group and ra...
Embodiment 3
[0056] Whole Exome Sequencing and Data Analysis
[0057] The inventors selected tumor tissue samples from 8 neoadjuvant chemoradiotherapy-sensitive patients and 7 neoadjuvant chemoradiotherapy-insensitive patients for whole exome sequencing screening.
[0058] The present invention adopts the whole exome sequencing method of Themor Fisher Company to screen differential gene mutation sites between neoadjuvant chemoradiotherapy-sensitive patients and neoadjuvant chemoradiotherapy-insensitive patients, and exome sequencing can detect the exon regions of all protein-coding genes Mutation frequency of DNA mutation sites. See the Themor Fisher website for specific steps. After calibration of the obtained raw data, the inventors used the Ion Reporter analysis method of the Ion Torrent information platform and selected differential gene DNA mutation sites, and finally screened and obtained 1385 candidate gene DNA mutation sites for subsequent verification.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com