Oxybutynin transdermal patch and preparation method thereof
A technology for transdermal plaster and ointment, which is applied in the directions of pharmaceutical formulations, medical preparations without active ingredients, and medical preparations containing active ingredients, etc. Harm skin health and other problems, to achieve the effect of prolonging the duration of the drug effect, prolonging the application time, and increasing the porosity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0038] The preparation method of described precrosslinked pectin is as follows: pectin is dispersed in water with the mass volume ratio of 1g:20-30mL, adds guava dialdehyde, and the mass ratio of described guava dialdehyde and pectin is 1: 25-35, after mixing evenly, crosslinking reaction at 50-60°C for 1-2h to obtain pre-crosslinked pectin.
[0039] The preparation method of the pectinase@ethylcellulose / chitosan microcapsules is as follows:
[0040](1) ethyl cellulose and chitosan that mass ratio is 9-12:1 are added in acetone, the mass volume ratio of described ethyl cellulose and acetone is 1g:70-80mL, after swelling, add pectin Enzymes, fully mixed to obtain a mixed solution;
[0041] (II) adding the mixed solution to the liquid paraffin containing Span-80, the volume ratio between the acetone in the step (I) and the liquid paraffin in the step (II) is 1:5-7, the Span-80 and The volume ratio of liquid paraffin is 1:60-70, stir until the acetone is completely volatilized,...
Embodiment 1
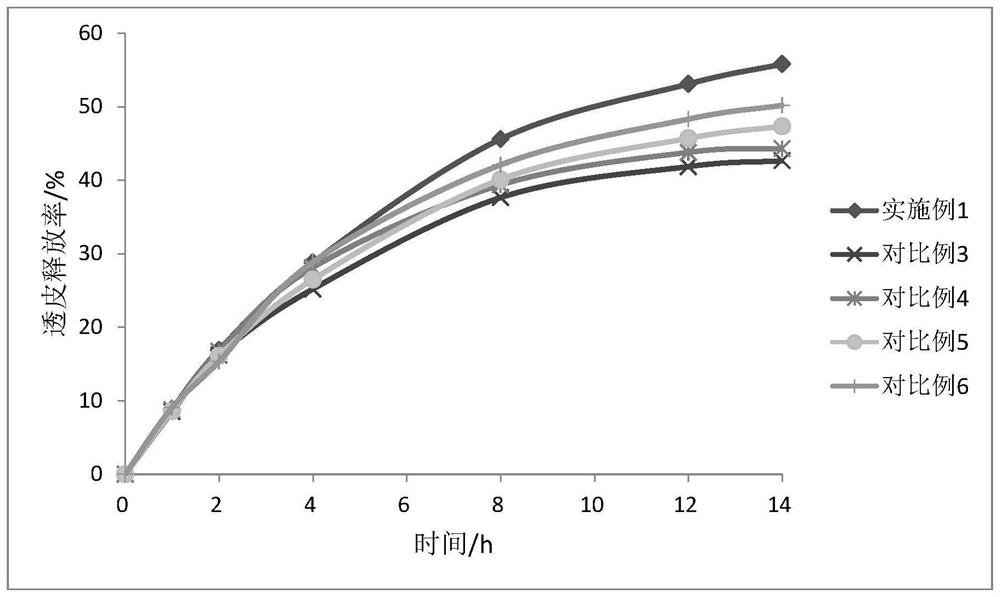
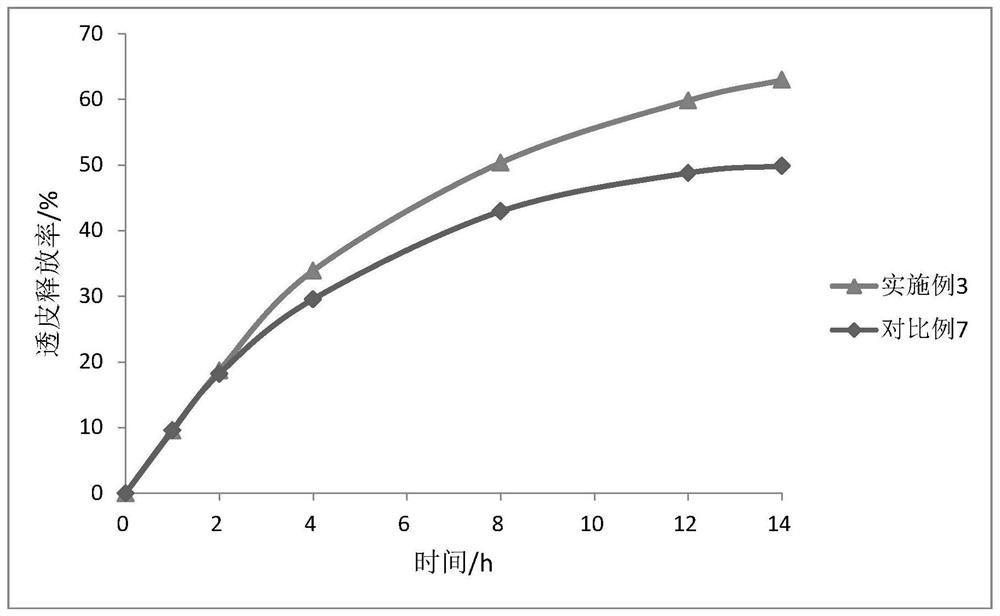
[0048] An oxybutynin transdermal plaster, comprising a backing layer and a medicated ointment, the medicated ointment includes the following raw materials in mass percentages: 3% oxybutynin hydrochloride, 15% sodium polyacrylate NP-700, pre- Crosslinked Pectin 5%, Hypromellose 1%, Aluminum Glycol 1%, Pectinase@Ethylcellulose / Chitosan Microcapsules 1.5%, Sorbitol 15%, Laurazone 1%, Ethanol 15%, water 42.5%.
[0049] A preparation method of the oxybutynin transdermal patch, comprising the following steps:
[0050] (1) Preparation of pre-crosslinked pectin: disperse pectin into water with a mass volume ratio of 1g:20mL, add guava dialdehyde, the mass ratio of guava dialdehyde to pectin is 1:25, mix well Afterwards, cross-linking reaction at 60°C for 1 hour to obtain pre-cross-linked pectin;
[0051] (2) Preparation of pectinase@ethylcellulose / chitosan microcapsules:
[0052] (2.1) Add ethyl cellulose and chitosan with a mass ratio of 12:1 into acetone, the mass volume ratio of...
Embodiment 2
[0059] An oxybutynin transdermal plaster, comprising a backing layer and a medicated ointment, the medicated ointment includes the following raw materials in mass percentages: oxybutynin hydrochloride 4%, sodium polyacrylate NP-700 13%, pre- Crosslinked Pectin 6%, Hypromellose 1.5%, Aluminum Glycol 0.8%, Pectinase@Ethylcellulose / Chitosan Microcapsules 2.5%, Sorbitol 13%, Laurazone 1.5%, Ethanol 13%, water 41.7%.
[0060] A preparation method of the oxybutynin transdermal patch, comprising the following steps:
[0061] (1) Preparation of pre-crosslinked pectin: disperse pectin into water with a mass volume ratio of 1g:25mL, add guava dialdehyde, the mass ratio of guava dialdehyde to pectin is 1:30, mix well Afterwards, cross-linking reaction at 55°C for 1.5 hours to obtain pre-cross-linked pectin;
[0062] (2) Preparation of pectinase@ethylcellulose / chitosan microcapsules:
[0063] (2.1) Add ethyl cellulose and chitosan with a mass ratio of 10:1 into acetone, the mass volume...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com