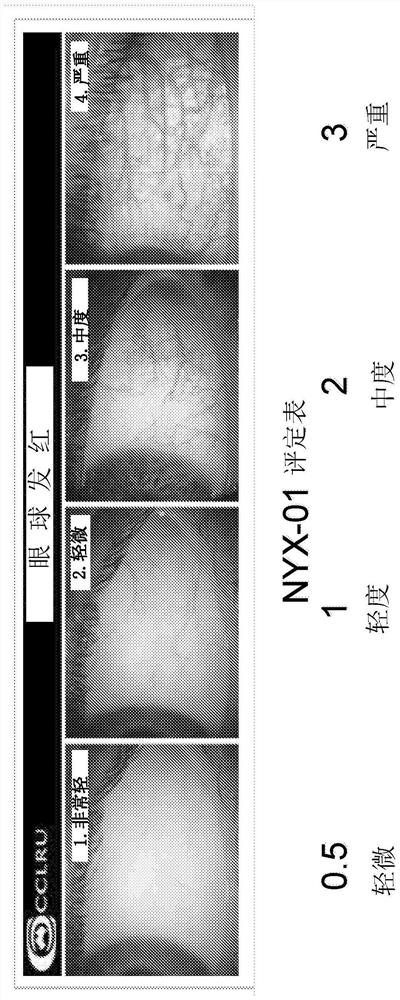
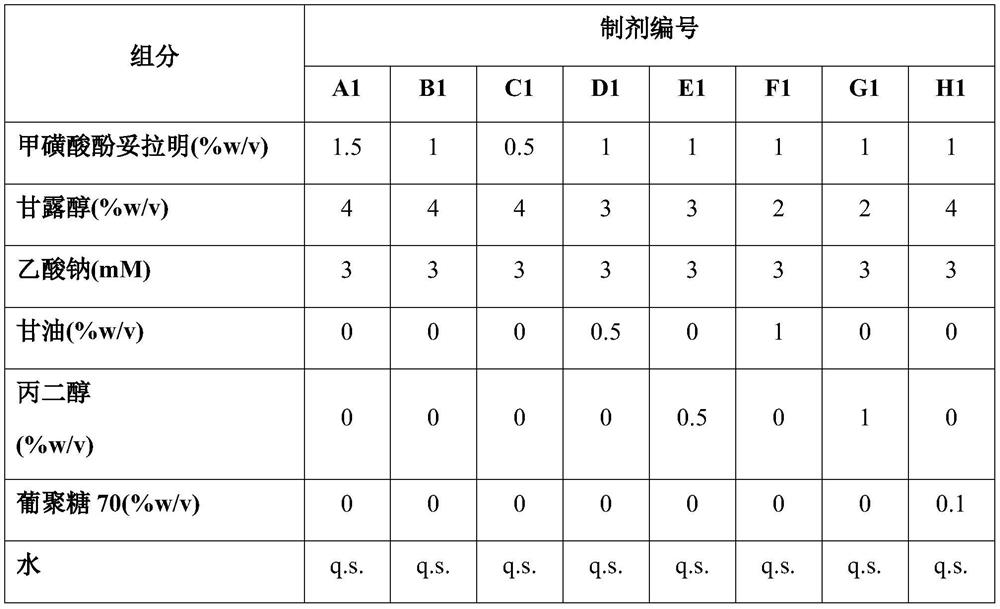
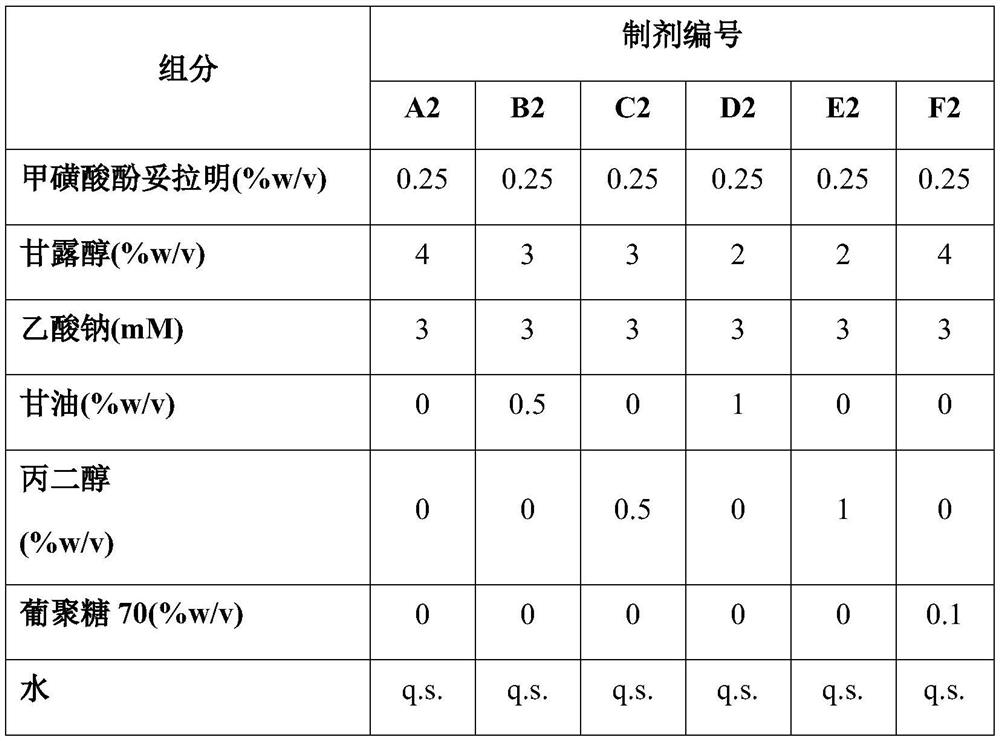
Methods and compositions for treatment of glaucoma and related conditions
A technology for glaucoma and disease, applied in the direction of drug combination, active ingredients of heterocyclic compounds, sensory diseases, etc., can solve problems such as lowering intraocular pressure, changing blood pressure, increasing intraocular pressure, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0394] Example 1 – Phentolamine mesylate reduces intraocular pressure in healthy human subjects with normal intraocular pressure
[0395] An aqueous solution containing phentolamine mesylate was administered as eye drops to the eyes of healthy persons with normal intraocular pressure, and the decrease in the intraocular pressure of the eyes was measured. The experimental procedure and results are described below.
[0396] Part I – Experimental Procedures
[0397] One of the test articles in Table 1 was administered in the form of eye drops to the eyes of healthy human subjects with normal intraocular pressure. The intraocular pressure in the eyes of the subjects was measured before the test article was applied and then again 2-3 hours after the test article was applied.
[0398] Table 1.
[0399] test product
describe
0.5%PM Phentolamine Mesylate 0.5% w / w in an aqueous vehicle 1% PM Phentolamine mesylate 1% w / w in aqueous vehicle pla...
Embodiment 2
[0407] Example 2 – Phentolamine mesylate reduces intraocular pressure in human subjects with an intraocular pressure of at least 22 mmHG
[0408] The ability of phentolamine mesylate to reduce intraocular pressure (IOP) in the eyes of human subjects with IOP ≥ 22 mmHg can be assessed based on clinical studies in which phentolamine mesylate containing Patients were then evaluated for the reduction in intraocular pressure in eyes receiving the aqueous ophthalmic solution containing phentolamine mesylate. The experimental procedure and results are described below.
[0409] Part I – Experimental Procedures
[0410] Human subjects are screened for potential enrollment and, if eligible, enrolled in the study. The inclusion and exclusion criteria of this study are as follows. If a subject does not meet the inclusion / exclusion criteria, but the investigator believes that the subject should be included in the study, deviations are allowed after discussions between the Principal In...
Embodiment 3
[0441] Example 3 - Phentolamine mesylate reduces intraocular pressure in human subjects with bilateral open-angle glaucoma or ocular hypertension
[0442] The ability of phentolamine mesylate to reduce intraocular pressure in the eyes of human subjects with bilateral open-angle glaucoma or ocular hypertension can be evaluated based on clinical studies in which phentolamine mesylate containing Aqueous ophthalmic solution of phentolamine mesylate was administered to patients, and then patients were evaluated for reduction in intraocular pressure in eyes receiving aqueous ophthalmic solution containing phentolamine mesylate. The experimental procedure and results are described below.
[0443] Part I – Experimental Procedures
[0444] Human subjects are screened for potential enrollment and, if eligible, enrolled in the study. The inclusion and exclusion criteria of this study are as follows.
[0445] Inclusion criteria
[0446] · Aged 18 years or older.
[0447] ·Diagnos...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com