Antagonistic cd40 monoclonal antibodies and uses thereof
An antibody, CDR2 technology, applied in the direction of antibodies, antibody medical components, anti-tumor drugs, etc., can solve the problems of limited stimulation potential and weak availability of anti-CD40 antibodies
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0205] Example 1: Binding of mouse anti-human CD40 antibody to human CD40
[0206] Mouse anti-human CD40 antibodies were raised and tested for binding to human CD40 by surface plasmon resonance (SPR). Table 8 shows the Vh and Vk sequences of each antibody.
[0207] Table 8
[0208] Mouse anti-human CD40 variable heavy and light chain sequences
[0209]
[0210]
[0211]
[0212]
[0213]
[0214]
[0215]
[0216] CD40 kinetics and avidity data for the binding of human CD40 monomers to mouse anti-human CD40 antibody captured on the protein A sensor chip surface were assessed by SPR. Data are shown in Table 9. Data shown are for a single concentration of CD40 analyte (1 μΜ) and are therefore reported as apparent (app) values.
[0217] Table 9
[0218] SPR kinetics / affinity data
[0219]
[0220] Based on SPR data and sequence data, three antibodies ADX_Y1258.ZZ0-1, ADX_Y1262.ZZ0-1 and ADX_Y1268.ZZ0-1 were selected for humanization.
Embodiment 2
[0221] Example 2: Humanization of Y12XX and selection of humanized variants
[0222] The humanization background / procedure was as discussed in the section "II. Engineered and Modified Antibodies" in WO2017004006, which is incorporated herein by reference in its entirety. Based on this analysis, nine (9) humanized Vh sequences (Vh-hz1, Vh-hz2, Vh-hz3, Vh-hz4, Vh-hz5, Vh-hz6, Vh-hz9, Vh-hz10 and Vh-hz11 were selected. ) and three (3) humanized VK sequences (Vk-hz1, Vk-hz2 and Vk-hz3) were tested. In addition, five (5) humanized Vh sequences (Vh-hz7, Vh-hz8, Vh-hz12, Vh-hz13 and Vh-hz14) were designed to contain mutations aimed at reducing the designed chemical liability risk. Mutations include D100Q (Y1262_IGHV1.6908-D100Q) and P101A (Y1262_IGHV1.6908-P101A) mutations to mitigate potential hydrolysis risk in Y1262_IGHV1.6908. Mutations also include N55Q (Y1268_IGHV1.6908-N55Q), G56A (Y1268_IGHV1.6908-G56A) and S54T-N55T double mutation (Y1268_IGHV1.6908-S54T-N55T) to alleviate...
Embodiment 3
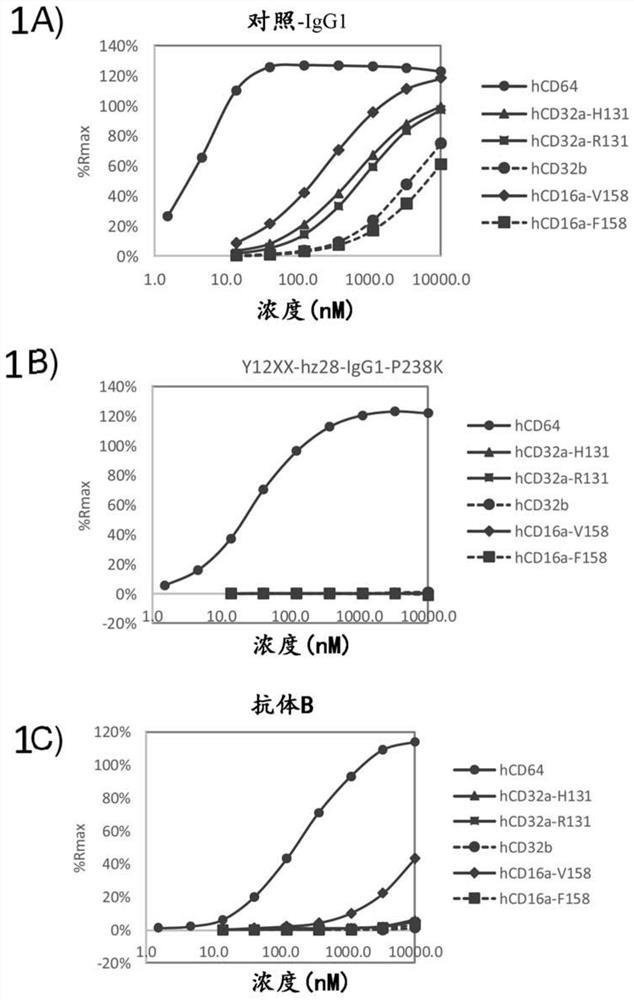
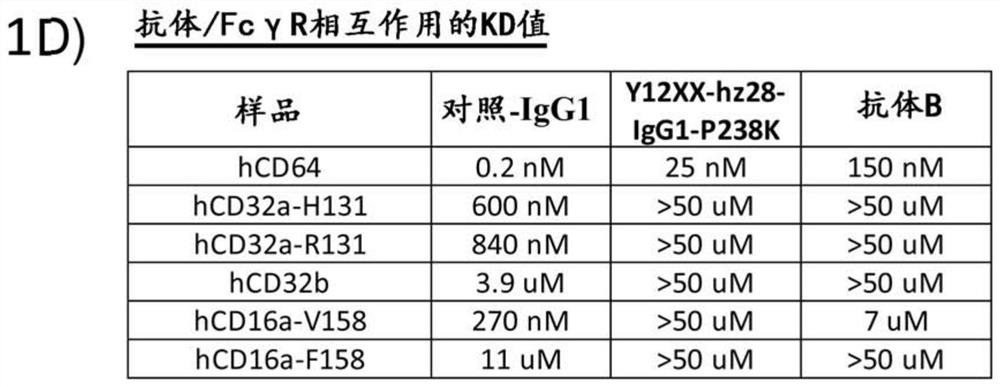
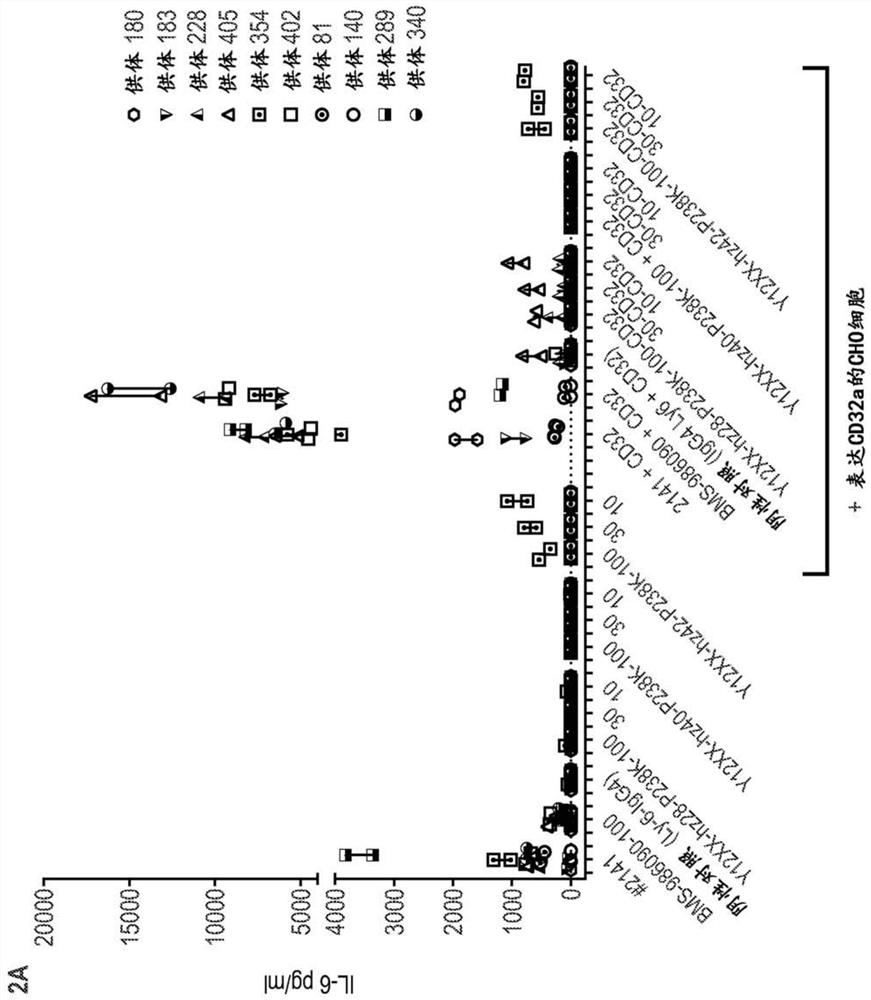
[0271] Example 3: In vitro Fc receptor analysis
[0272] Antibodies can exert effector functions, such as complement-dependent cytotoxicity (CDC) and antibody-dependent cellular cytotoxicity (ADCC), by binding the Fc region to Fcγ receptors (FcγR) or complement factors on the surface of immune cells. Antibody-dependent phagocytosis is another potential Fc effector function. To further characterize the properties of the humanized Y12XX antibody, the complement-dependent cytotoxicity (CDC), antibody-dependent cellular phagocytosis (ADCP) and antibody-dependent cellular cytotoxicity (ADCC) of the antibody were analyzed. Table 15 lists the antibodies analyzed in this example.
[0273] Table 15
[0274]
[0275] CDC analysis was performed as follows. "CDC assay medium" refers to Roswell Park Memorial Institute medium (RPMI) 1640 (HyClone) with L-glutamine, without phenol red (HyClone), supplemented with 0.1% BSA (Sigma) and 1% penicillin-streptomycin (Life Technologies). Fi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com