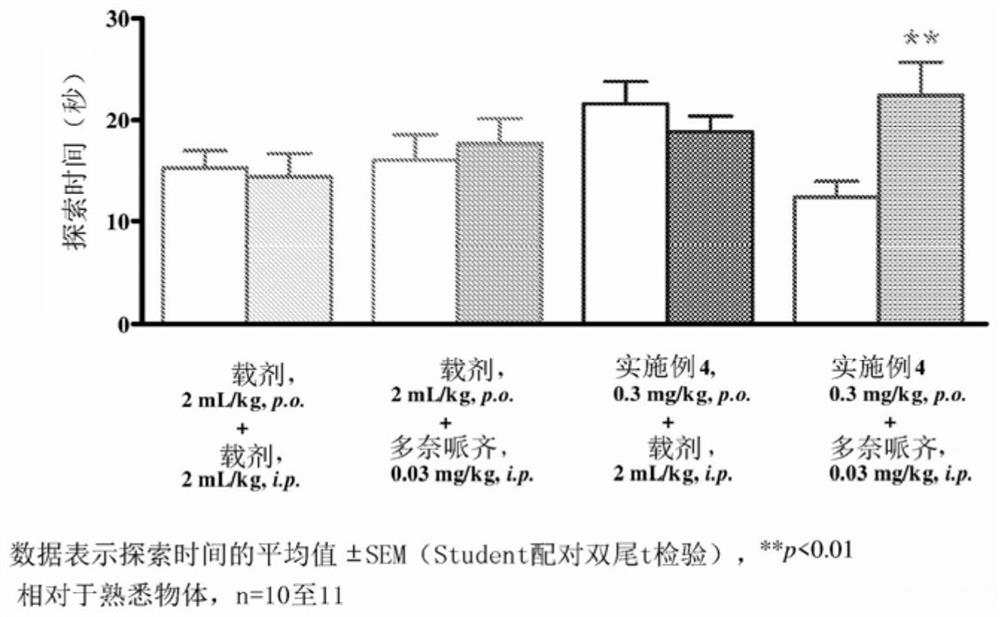
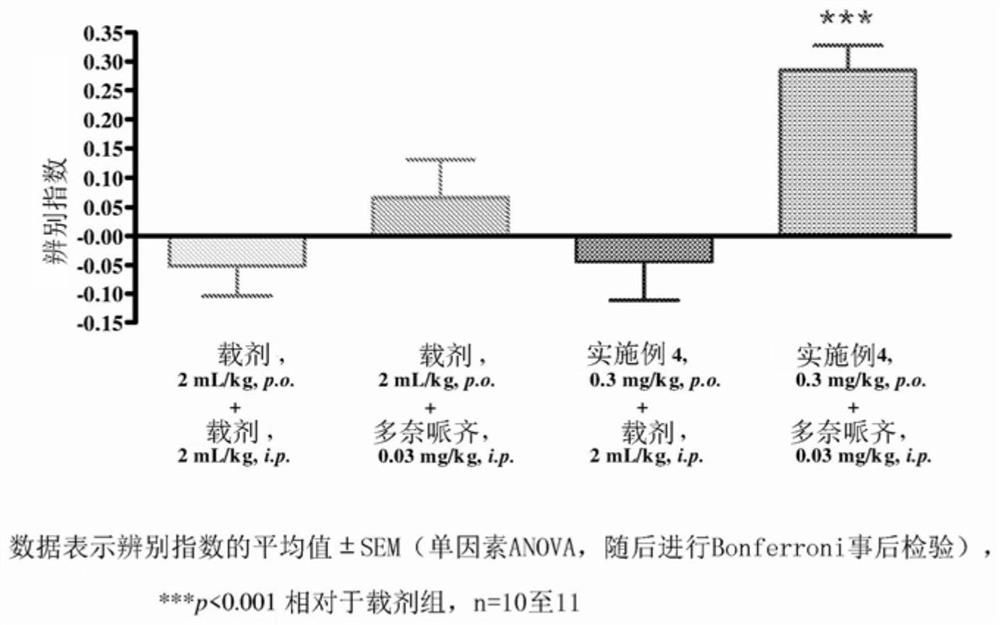
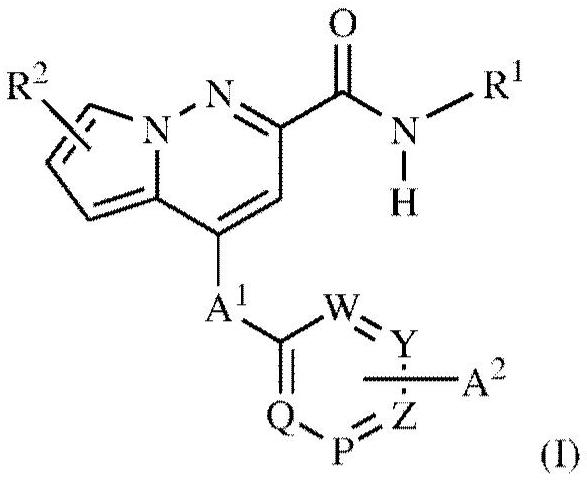
Pyrrolo-pyridazine derivatives as muscarinic m1 receptor positive allosteric modulators
A technology of pyridazine and pyrrole, which is applied in anti-inflammatory agents, drug combinations, non-central analgesics, etc., and can solve problems such as unreleased drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0267] Example 1: N-[1-hydroxy-cyclohexylmethyl]4-(4-chlorobenzyl)-pyrrolo[1,2-b]pyridazine-2-carboxamide
[0268]
[0269] Step-1: To a stirred slurry of potassium tert-butoxide (4.31 g, 38.48 mmol) in anhydrous toluene (60.0 mL) cooled at 0° C. was added 1-(4-chlorophenyl) over a period of 15 minutes - A mixture of 2-propanone (5.0 g, 29.6 mmol) and diethyl oxalate (4.82 mL, 35.52 mmol). After stirring the reaction mixture at 0 °C for 2 hours, the reaction temperature was raised to RT and stirred for 16 hours. The reaction mass was cooled to ice bath temperature and quenched by adding aqueous acetic acid until the reaction pH reached 2.5. The reaction mass was diluted with EtOAc and the two layers were separated. The organic layer was washed with brine solution, washed with anhydrous Na 2 SO 4 Dry, and remove the solvent under reduced pressure to obtain the product of Step 1, ethyl 5-(4-chlorophenyl)-2,4-dioxopentanoate (7.6 g), in 96% yield.
[0270] 1 H-NMR (400MH...
Embodiment 2 to 18
[0277] Examples 2 to 18: The compounds of Examples 2 to 18 were prepared by following the experimental procedure as described in Example 1 with some non-critical changes.
[0278]
[0279]
[0280]
[0281]
[0282]
[0283]
Embodiment 19
[0284] Example 19: N-[2-Hydroxy-2-methyl-propyl]4-[4-(1-methyl-1H-pyrazol-4-yl)-benzyl]-pyrrolo[1,2 -b]pyridazine-2-carboxamide
[0285]
[0286] Step-1: To a stirred slurry of potassium tert-butoxide (6.84 g, 61.01 mmol) in anhydrous toluene (93.0 mL) cooled at 0°C was added 1-(4-bromophenyl) over a period of 15 minutes - A mixture of 2-propanone (10.0 g, 46.93 mmol), diethyl oxalate (7.64 mL, 56.31 mmol) in toluene (93.0 mL). After stirring the reaction mixture at 0 °C for 2 hours, the reaction temperature was raised to RT and stirred at this temperature for 16 hours. The reaction mass was cooled to ice bath temperature and a solution of acetic acid (5.63 mL) and water (46.9 mL) was added until the reaction pH reached 5. The reaction mass was diluted with EtOAc and the two layers were separated. The organic layer was washed with brine solution, washed with anhydrous Na 2 SO 4 Dry and remove the solvent under reduced pressure to obtain the product of Step 1, ethyl 5-(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com