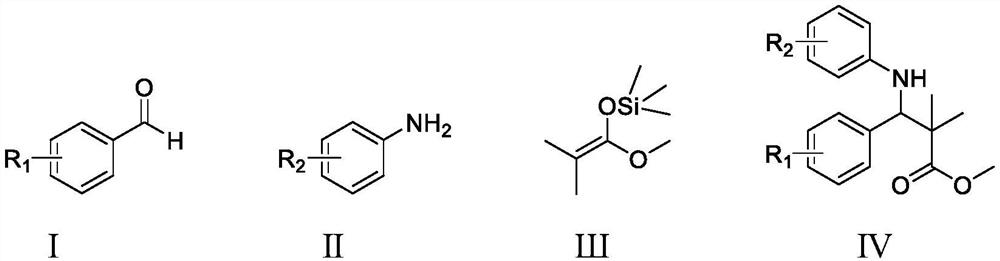
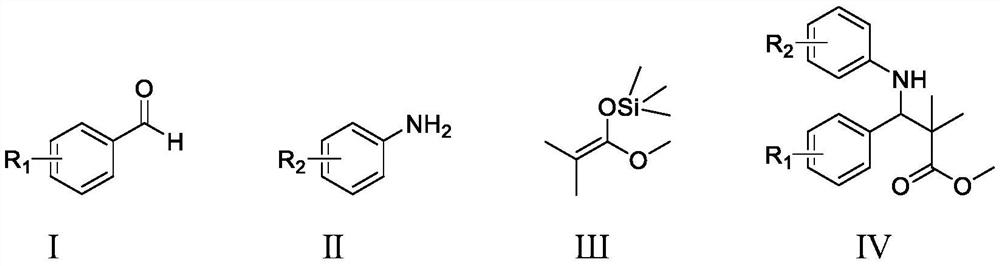
Method for catalytically synthesizing beta-amino carboxylic ester through cooperation of pentamethyl titanocene trichloride and 4-hydroxybenzoic acid
A technology of pentamethyltrichlorotitanocene and hydroxybenzoic acid, which is applied in the field of synthesis of β-aminocarboxylates, can solve the problems of catalyst prefabrication, harsh reaction conditions, and low reaction efficiency, and achieve a wide range of biological activities and Medicinal value, mild reaction conditions, and high atom economy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
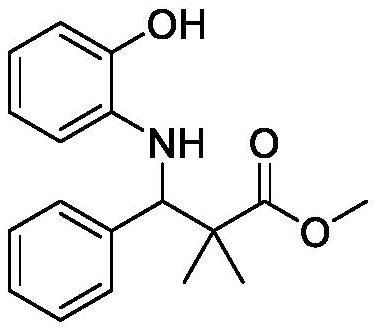
[0015] Synthetic structural formula following 2,2-dimethyl-3-phenyl-3-(2-hydroxyl) methyl phenylalanine
[0016]
[0017] Add 0.0545g (0.5mmol) o-aminophenol, 71μL (0.7mmol) benzaldehyde, 102μL (0.5mmol) silyl ketene acetal, 0.0125g (0.05mmol) titanocene dichloride, 0.0181g (0.1mmol) p-hydroxybenzoic acid, 0.5mL ethanol, stirred and reacted at room temperature for 12 hours, stopped the reaction, cooled to room temperature naturally, removed ethanol by rotary evaporation, and separated with a silica gel column (eluent was ethyl acetate and sherwood oil) The volume ratio of the mixture is 1:10) to obtain methyl 2,2-dimethyl-3-phenyl-3-(2-hydroxy)phenylalanine with a yield of 93%.
[0018] The resulting product was characterized by a Bruker Avance superconducting Fourier digital NMR spectrometer, and the characterization data were: 1 HNMR (400MHz, CDCl 3 )δ7.36–7.23(m,5H),6.78–6.48(m,3H),6.43(d,J=6.9Hz,1H),5.73(s,1H),4.98(s,1H),4.62(s ,1H), 3.73(s,3H), 1.29(s,3H), 1.26(s, 3...
Embodiment 2
[0020] Synthesis of 2,2-dimethyl-3-(4-nitro)phenyl-3-(2-hydroxyl) methyl phenylalanine with the following structural formula
[0021]
[0022] In Example 1, the benzaldehyde used is replaced with equimolar 4-nitrobenzaldehyde, and other steps are the same as in Example 1 to obtain 2,2-dimethyl-3-(4-nitro)phenyl- 3-(2-Hydroxy)phenylalanine methyl ester, the yield was 66%.
[0023] The resulting product was characterized by a Bruker Avance superconducting Fourier digital NMR spectrometer, and the characterization data were: 1 H NMR (400MHz, CDCl 3 )δ8.17(d, J=8.6Hz, 2H), 7.51(d, J=8.6Hz, 2H), 7.28(s, 1H), 6.72(d, J=7.6Hz, 1H), 6.64(t, J=7.5Hz, 1H), 6.56(t, J=7.4Hz, 1H), 6.29 (d, J=7.7Hz, 1H), 5.46(s, 1H), 5.14(s, 1H), 4.69(s, 1H), 3.73(s,3H), 1.32(s,3H), 1.28(s,4H), 1.26(s,4H).; 13 C NMR (101MHz, CDCl 3 )δ176.63, 147.36, 143.68, 134.96, 129.20, 123.2, 121.28, 118.15, 114.37, 113.00, 64.21, 52.40, 47.09, 29.66, 24.15, 20.61.
Embodiment 3
[0025] Synthetic structural formula following 2,2-dimethyl-3-p-chlorophenyl-3-(2-hydroxyl) methyl phenylalanine
[0026]
[0027] In Example 1, the benzaldehyde used was replaced with 4-chlorobenzaldehyde, and other steps were the same as in Example 1 to obtain 2,2-dimethyl-3-p-chlorophenyl-3-(2-hydroxy)benzene Methyl allanate, the yield was 89%.
[0028] The resulting product was characterized by a Bruker Avance superconducting Fourier digital NMR spectrometer, and the characterization data were: 1 H NMR (400MHz, CDCl 3 )δ7.27(t, J=3.2Hz, 4H), 6.72(d, J=7.5Hz, 1H), 6.66(t, J=7.4Hz, 1H), 6.56(t, J=7.3Hz, 1H) ,6.36(d,J=7.7Hz,1H),4.57(s,1H),3.72(s,3H),1.28(s,3H),1.24(s,3H); 13 C NMR (101MHz, CDCl 3 )δ177.38, 144.08, 137.78, 135.37, 133.24, 129.70, 128.24, 121.2, 118.07, 114.36, 113.68, 64.11, 52.33, 47.27, 24.29, 20.24.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com