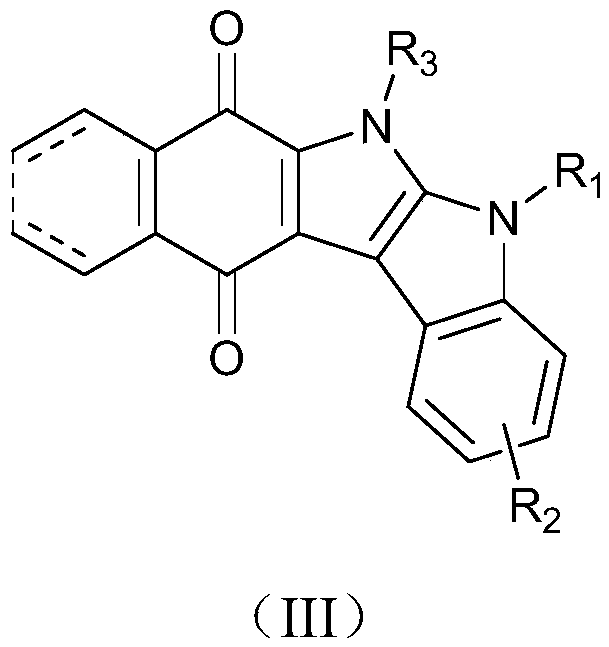
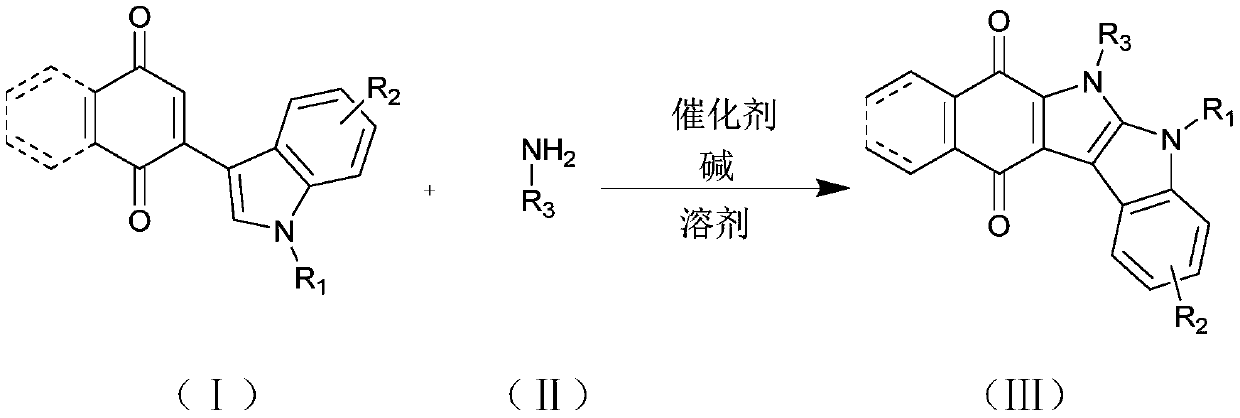
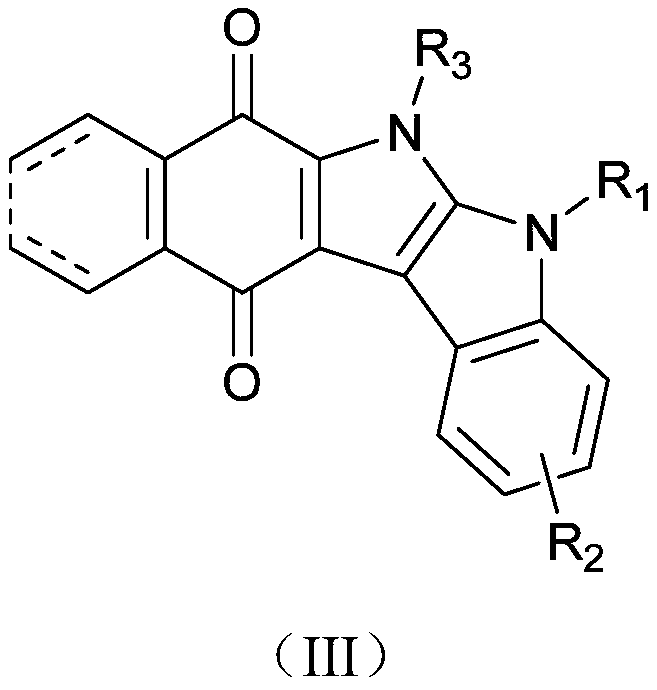
Carbazole indolequinone derivative as well as preparation method and application thereof
A technology of carbazole indole quinone and its derivatives, which is applied in the field of carbazole indole quinone derivatives and its preparation, can solve problems affecting industrial production, harsh reaction conditions, and low product yield, and achieve good fluorescence characteristics, The effect of simple operation and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038]
[0039] Add nitrogen methyl indole naphthoquinone compound (86.2mg, 0.3mmol), aniline (41.9mg, 0.45mmol), CoCl 2(1.2mg, 0.009mmol), t-BuOK (50.5mg, 0.45mmol) and DMF (2mL), mix well. Then, it was stirred at 120° C. for 24 h under an air atmosphere. After the reaction was complete (monitored by TLC), it was cooled to room temperature. The reaction was quenched with saturated brine (5 ml), and the mixture was extracted with EtOAc (3 x 5 mL). Then the aqueous layer was extracted with ethyl acetate, the organic layers were combined, dried over anhydrous magnesium sulfate, filtered, concentrated, and separated by column chromatography (PE:EA=5:1) to obtain a red solid 3aa with a yield of 89%.
[0040] The melting point is 274-276°C. 1H NMR (400MHz, CDCl 3 )δ8.35(d, J=7.3Hz, 1H), 8.14(d, J=5.9Hz, 1H), 7.94(d, J=5.5Hz, 1H), 7.60(d, J=15.9Hz, 7H) ,7.36–7.23(m,3H),7.17(d,J=7.6Hz,1H),3.24(s,3H).13C NMR(101MHz,CDCl 3 )δ181.88, 174.08, 143.50, 136.44, 134.64, 133.08, 132....
Embodiment 2
[0042]
[0043] Add nitrogen methyl indole naphthoquinone compound (86.2mg, 0.3mmol), p-methylaniline (48.2mg, 0.45mmol), CoCl 2 (1.2mg, 0.009mmol), t-BuOK (50.5mg, 0.45mmol) and DMF (2mL), mix well. Then, it was stirred at 120° C. for 24 h under an air atmosphere. After the reaction was complete (monitored by TLC), it was cooled to room temperature. The reaction was quenched with saturated brine (5 ml), and the mixture was extracted with EtOAc (3 x 5 mL). Then the aqueous layer was extracted with ethyl acetate, the organic layers were combined, dried over anhydrous magnesium sulfate, filtered, concentrated, and separated by column chromatography (PE:EA=5:1) to obtain a red solid 3ab with a yield of 85%.
[0044] The melting point is 275-277°C. 1H NMR (400MHz, CDCl 3 )δ8.36(d,J=7.2Hz,1H),8.16(s,1H),7.97(s,1H),7.59(s,2H),7.46(s,2H),7.41(s,2H), 7.33–7.27(m,2H),7.20(d,J=7.5Hz,1H),3.28(s,3H),2.51(s,3H).13C NMR(101MHz,CDCl 3 )δ181.92,174.12,143.54,139.93,134.73,133.76,133....
Embodiment 3
[0046]
[0047] Add nitrogen methyl indole naphthoquinone compound (86.2mg, 0.3mmol), p-methoxyaniline (55.4mg, 0.45mmol), CoCl 2 (1.2mg, 0.009mmol), t-BuOK (50.5mg, 0.45mmol) and DMF (2mL), mix well. Then, it was stirred at 120° C. for 24 h under an air atmosphere. After the reaction was complete (monitored by TLC), it was cooled to room temperature. The reaction was quenched with saturated brine (5 ml), and the mixture was extracted with EtOAc (3 x 5 mL). Then the aqueous layer was extracted with ethyl acetate, and the organic layers were combined, dried over anhydrous magnesium sulfate, filtered, concentrated, and separated by column chromatography (PE:EA=5:1) to obtain a red solid 3ac with a yield of 81%.
[0048] The melting point is 266-268°C. 1H NMR (400MHz, CDCl 3 )δ8.34(d, J=7.1Hz, 1H), 8.15(s, 1H), 7.95(s, 1H), 7.58(s, 2H), 7.51(d, J=7.5Hz, 2H), 7.35– 7.26(m,2H),7.17(d,J=7.5Hz,1H),7.10(d,J=7.6Hz,2H),3.93(s,3H),3.28(s,3H).13C NMR(101MHz, CDCl 3 )δ181.88, 174...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com