TMEM101 Gene Methylation Detection Kit for Early Screening of Endometrial Cancer in Human Peripheral Blood Circulating Tumor DNA
A technology for endometrial cancer and human peripheral blood, applied in biochemical equipment and methods, microbial determination/inspection, instruments, etc., can solve the problem of untraceable blood early screening for endometrial cancer, long detection time, and cumbersome process and other problems, to achieve the effect of good patient compliance, easy operation and simple interpretation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
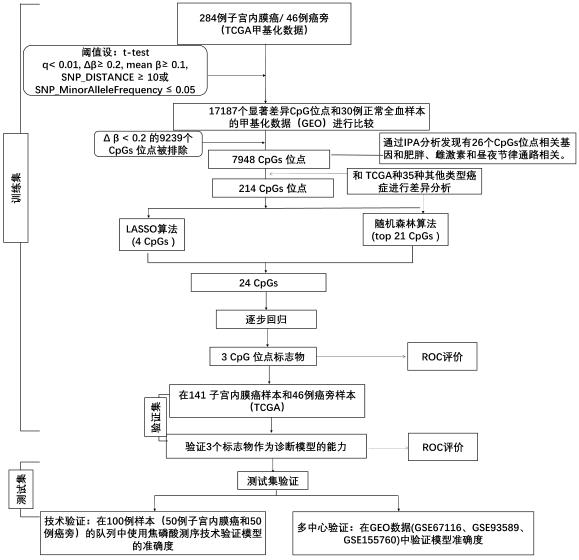
[0070] The difficulty of the present invention is to find the site for distinguishing endometrial cancer from other tumors. In order to lock the site for distinguishing endometrial cancer and other tumors proposed in the present invention, more than 33 tumors in the database were used in the research and development process. The methylation numbers of nearly 13,000 tumors have been developed, and the analysis process has been developed by itself, and a large amount of tumor tissue data has been actually detected by pyrosequencing. Some results are displayed as figure 1 As shown, specifically:
[0071] 1. In this patent, in the process of locking candidate regions, the idea of marker screening is divided into two parts: 1. Training set, 2. Test set
[0072] 1. Training set
[0073] 1.1 Using the 450K chip methylation data of 284 cases of endometrial cancer (2 / 3) and 46 cases of adjacent cancers in TCGA, after data preprocessing, difference analysis was performed, and the th...
Embodiment 2
[0089] Materials: Plasma samples to be tested, positive quality control products, and negative quality control products. The positive quality control product is fully methylated human genomic DNA modified by bisulfite, and the negative quality control product is unmethylated human genomic DNA modified by bisulfite.
[0090] TMEM101 gene methylation detection kit in human peripheral blood circulating tumor DNA, including: TMEM101 gene target site methylation-specific primers; TMEM101 gene-specific hydrolysis probes; internal reference gene primers and internal reference gene hydrolysis probes ;
[0091] The target site of the TMEM101 gene is at least one CpG site in the interval of 2000 bp upstream and downstream of the transcription initiation site of TMEM101; the internal reference gene is one or more of GAPDH and β-actin.
[0092] The methylation-specific primers for the target site of the TMEM101 gene (the final concentration is: 0.1-1 µM) include:
[0093] Forward primer...
Embodiment 3
[0133] Through the cfDNA detection of 20 cases of healthy controls and 26 samples of Shanghai xx Hospital (refer to Example 1 for the detection method), the detection sensitivity of the kit of the present invention is 87%, the specificity is 100%, and the accuracy rate is 96%. See Table 5 and Table 6.
[0134] Table 5 Comparison of clinicopathological information and experimental results of 26 samples from xx Hospital in Shanghai.
[0135]
[0136] Table 6 The detection results of the regions involved in the present invention in 46 samples.
[0137]
[0138] As can be seen from Table 5 and Table 6, the detection kit of the present invention has reliable detection results, high sensitivity, good specificity, high accuracy, simple interpretation, and can trace the source of endometrial cancer tissue, reaching the level of the prior art. Unforeseen technical effects.
[0139] In summary, the kits or reagents of the embodiments of the present invention have the following a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com